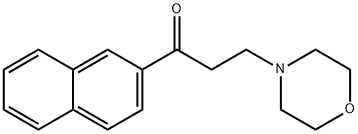
3-(morpholin-4-yl)-1-(naphthalen-2-yl)propan-1-one synthesis
- Product Name:3-(morpholin-4-yl)-1-(naphthalen-2-yl)propan-1-one
- CAS Number:27478-04-2
- Molecular formula:C17H19NO2
- Molecular Weight:269.34
Yield:27478-04-2 41%
Reaction Conditions:
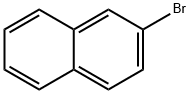
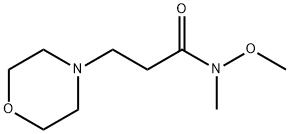
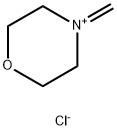
with tert.-butyl lithium in tetrahydrofuran;pentane;Inert atmosphere;Weinreb Ketone Synthesis;
Steps:
17 General procedure for the preparation of compounds 7-30
General procedure: Under argon atmosphere, tert-butyllithium (2.2 equiv., 1.9M inpentane) was added dropwise to a -78° C cooled solution of theappropriate arylbromide (1.5 equiv.) in anhydrous THF. After20 min, the solution of the corresponding Weinreb amide in anhydrous THF was added dropwise. The stirring was continued for5 additional hours and then quenched with water. The reactionwasextracted with Et2O (2 5 mL) and washed with water (5 mL) andbrine (10 mL). The organic phase was dried (anhydrous Na2SO4),filtered and, after removal of the solvent under reduced pressure,the so-obtained crude mixture was subjected to chromatography(silica gel) to afford pure compounds. Lastly, pure compounds wereconverted into their corresponding hydrochlorides, adding anethereal solution of HCl (1.0 equiv., 1M in Et2O).
References:
Rui, Marta;Rossino, Giacomo;Coniglio, Stefania;Monteleone, Stefania;Scuteri, Arianna;Malacrida, Alessio;Rossi, Daniela;Catenacci, Laura;Sorrenti, Milena;Paolillo, Mayra;Curti, Daniela;Venturini, Letizia;Schepmann, Dirk;Wünsch, Bernhard;Liedl, Klaus R.;Cavaletti, Guido;Pace, Vittorio;Urban, Ernst;Collina, Simona [European Journal of Medicinal Chemistry,2018,vol. 158,p. 353 - 370]

50-00-0
858 suppliers
$10.00/25g
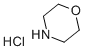
10024-89-2
122 suppliers
$5.00/5g
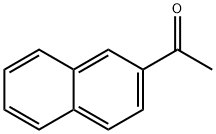
93-08-3
409 suppliers
$6.00/25g

27478-04-2
3 suppliers
inquiry
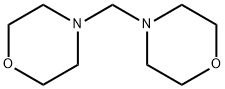
5625-90-1
148 suppliers
$15.00/5g

27478-04-2
3 suppliers
inquiry