
3-(N-CyclopropylsulfaMoyl)benzoic acid synthesis
- Product Name:3-(N-CyclopropylsulfaMoyl)benzoic acid
- CAS Number:852933-50-7
- Molecular formula:C10H11NO4S
- Molecular Weight:241.26
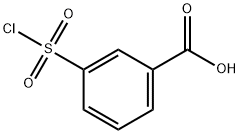
4025-64-3
204 suppliers
$6.00/1g
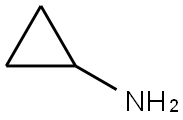
765-30-0
484 suppliers
$10.00/25g

852933-50-7
20 suppliers
$57.50/250mg
Yield:852933-50-7 55%
Reaction Conditions:
Stage #1: 3-Carboxybenzenesulfonyl chloride;Cyclopropylaminewith triethylamine in tetrahydrofuran at 0 - 20; for 3 h;
Stage #2: with hydrogenchloride in tetrahydrofuran;water; pH=3;
Steps:
1
3-(N-cylcopropylaminosulfonyl)benzoic acid (2.2):Cyclopropyl amine (0.26 g, 4.53 mmol) and triethyl amine (1.38 g, 13.6 mmol) are dissolved in tetrahydrofuran (15 ml). To this solution is added dropwise a tetrahydrofuran solution (5 mL) of 3-(chlorosulfonyl)benzoic acid (1 g, 4.53 mmol, subject compound 2.1) at 0°C. After adding, the mixture is heated up to room temperature, and stirred at room temperature for 3 h. After the reaction is completed as indicated by TLC, the reaction mixture is poured into 30 mL of saturated saline, and treated with 1 N hydrochloric acid to pH = 3. The mixture is extracted with ethyl acetate (20 mL x 2), and the organic phase is washed with saturated saline, dried over anhydrous sodium sulfate. The solvent is removed under reduced pressure to obtain a light white solid (subject compound 2.2, 0.6 g, 55%).
References:
WO2012/171488,2012,A1 Location in patent:Page/Page column 19-20