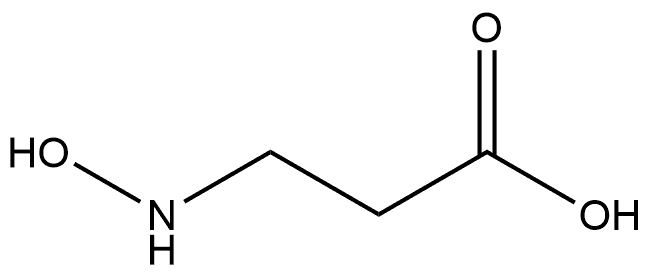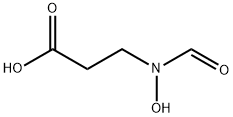
3-(N-Hydroxyformamido)propanoic acid synthesis
- Product Name:3-(N-Hydroxyformamido)propanoic acid
- CAS Number:1267521-41-4
- Molecular formula:C4H7NO4
- Molecular Weight:133.1
Yield:1267521-41-4 80%
Reaction Conditions:
Stage #1: formic acidwith acetic anhydride at 20; for 0.5 h;
Stage #2: 3-(N-hydroxyamino)propanoic acid at 0 - 20;
Steps:
3-(N-Formyl-N-hydroxyamino)propanoic acid (12)
A mixture of formic acid (0.2 mL, 2.25 mmol) and acetic anhydride (22 μL, 0.22 mmol) was stirred at room temperature. After 30 min a solution of 13 (25 mg, 0.18 mmol) in formic acid (0.5 mL) was added at 0°C and the reaction mixture was stirred overnight at room temperature. The reaction mixture was quenched with water and solvent was removed under pressure. The residue was purified by reverse-phase HPLC (C18), using H2O and acetonitrile containing 0.1% formic acid to yield pale yellow oil (25 mg, 80%) in a 7:4 mixture of amide rotamers. 1H NMR (500 M Hz, CD3OD) δ major rotamer 7.97 (s, 1H), 3.76 (t, J=6.0 Hz, 2H), 2.60 (t, J = 6.5 Hz, 2H); minor rotamer 8.25 (s, 1H), 3.82 (t, J = 6.0 Hz, 2H ), 2.66 (t, J = 6.5 Hz, 2H); 13C NMR (125 M Hz, CD3OD) δ 174.8, 163.7, 159.7, 47.3, 45.1, 37.1, 32.4.
References:
Tibrewal, Nidhi;Elliott, Gregory I. [Bioorganic and Medicinal Chemistry Letters,2011,vol. 21,# 1,p. 517 - 519] Location in patent:supporting information; experimental part