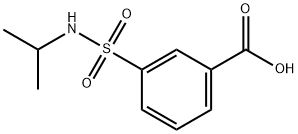
3-(N-IsopropylsulfaMoyl)benzoic acid synthesis
- Product Name:3-(N-IsopropylsulfaMoyl)benzoic acid
- CAS Number:716358-46-2
- Molecular formula:C10H13NO4S
- Molecular Weight:243.28
4025-64-3
207 suppliers
$6.00/1g
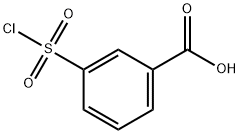
75-31-0
413 suppliers
$14.00/25mL

716358-46-2
15 suppliers
$45.00/100mg
Yield:-
Reaction Conditions:
in ethyl acetate at 25; for 3 h;
Steps:
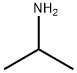
Compound 73 To an iced-cooled solution of 3-(chlorosulfonyl)benzoic acid (50.0 g, 226.6 mmol) in ethylacetate (1000 mL) was added isopropylamine (67.0 g, 1.13 mol) in one portion. The reaction mixture was stirred at 25°C for 3 hours. The resulting mixture was diluted with IN HC1 (500 mL) and extracted with ethyl acetate (2 x 500 mL). The combined organic layers were washed with brine (400 mL), dried over anhydrous Na2S04 and concentrated under reduced pressure resulting 3-(N-isopropylsulfamoyl)benzoic acid (46 g). To an ice-cooled mixture of 3-(N-isopropylsulfamoyl)benzoic acid (7.0 g, 28.77 mmol), 4-fiuoro-3-methylaniline (3.6 g, 28.77 mmol) and DIPEA (18.6 g, 143.91 mmol) in CH2C12 (70 mL) HATU (12.0 g, 31.56 mmol) was added under N2 atmosphere. The resulting mixture was stirred at 20° for 16 hours. The solvent was removed in vacuo. The mixture was washed with saturated aqueous critic acid (30 mL), brine (20 mL) and dried over Na2S04. The solvent was removed in vacuo. The residue was purified by preparative high performance liquid chromatography on SYNERGI 250*50 lOum (eluent: CH3CN in H20 (0.05% TFA) from 35% to 65%, v/v). The pure fractions were collected and adjusted to pH=7 with Amberlite IRA-900(OH) anionic exchange resin. The resin was filtered off. The filtrate was lyophilized to dryness resulting in compound 73 (7.5 g). Method B; Rt: 3.44 min. m/z : 351.1 (M+H)+ Exact mass: 350.1 1H NMR (400 MHz, DMSO-d6) δ ppm 10.49 (1 H, br. s), 8.36 (1 H, t, J=1.5 Hz), 8.19 (1 H, ddd, J=7.8, 1.5, 1.0 Hz), 8.01 (1 H, ddd, J=7.8, 1.5, 1.0 Hz), 7.76 (1 H, t, J=7.8 Hz), 7.68 (1 H, dd, J=7.0, 3.0 Hz), 7.75 (1 H, bs), 7.59 (1 H, ddd, J=9.0, 4.5, 3.0 Hz), 7.15 (1 H, t, J=9.0 Hz), 3.14 - 3.33 (1 H, m), 2.25 (3 H, d, J=1.5 Hz), 0.96 (6 H, d, J=6.5 Hz).
References:
WO2014/33170,2014,A1 Location in patent:Page/Page column 54; 55