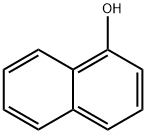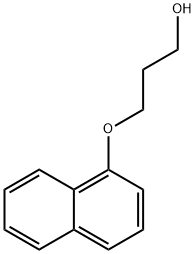
3-(naphthalen-1-yloxy)propan-1-ol synthesis
- Product Name:3-(naphthalen-1-yloxy)propan-1-ol
- CAS Number:54804-70-5
- Molecular formula:C13H14O2
- Molecular Weight:202.25
Yield:54804-70-5 79%
Reaction Conditions:
Stage #1: α-naphtholwith potassium carbonate in N,N-dimethyl-formamide; for 0.0833333 h;Inert atmosphere;
Stage #2: 1-bromo-3-propanol in N,N-dimethyl-formamide at 80; for 4 h;Inert atmosphere;
Steps:
3-(Naphthalene-1-yloxy)propane-1-ol,S8
Potassium carbonate (1.19 g; 8.61 mmol; 4 eq.)was added to a solution of 1-naphthol (0.934 g; 6.47 mmol; 3 eq.) dissolved inanhydrous DMF (40 mL) under a N2 atm. After 5 minutes 3-bromopropane-1-ol(0.19 mL; 2.16 mmol; 1 eq.) was added dropwise and the reactionmixture was heated to 80 oC for 4 hours. When the reaction had runto completion it was quenched with H2O and the aqueous phase was extractedwith ethyl acetate. The combined organic phases were washed with H2Oand brine, dried over magnesium sulfate, filtered and evaporated. The productwas purified by column chromatography (first pentane/ethyl acetate 8:1 thenpentane/ethyl acetate 2:1) and isolated as red oil in 79 % yield (0.343 g; 1.70mmol). Rf (pentane/ethyl acetate 2:1) 0.29. 1H-NMR (400MHz, CDCl3) δH 8.27 - 8.19 (m, 1H), 7.84 - 7.76 (m, 1H),7.52 - 7.34 (m, 4H), 6.84 (dd, J = 7.5, 1.0 Hz, 1H), 4.31 (t, J= 5.9 Hz, 2H), 3.98 (t, J = 6.0 Hz, 2H), 2.20 (quint, J =4.0 Hz, 2H), 1.70 (bs, 1H) ppm. 13C-NMR (100 MHz, CDCl3)δC 154.6, 134.6, 127.7, 126.5, 126.0, 125.7, 125.4, 121.9, 120.5,104.8, 65.7, 60.7, 32.2 ppm. HRMS (ES+): Calc. for C13H14O2K:m/z 225.0891; found m/z 225.0890.
References:
Brink?, Anne;Larsen, Maja T.;Kolds?, Heidi;Besenbacher, Louise;Kolind, Anders;Schi?tt, Birgit;Sinning, Steffen;Jensen, Henrik H. [Bioorganic and Medicinal Chemistry,2016,vol. 24,# 12,p. 2725 - 2738] Location in patent:supporting information