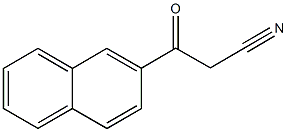
3-naphthalen-2-yl-3-oxo-propanenitrile synthesis
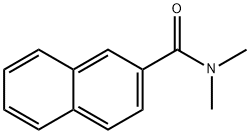
- Product Name:3-naphthalen-2-yl-3-oxo-propanenitrile
- CAS Number:613-57-0
- Molecular formula:C13H9NO
- Molecular Weight:195.22
Yield:613-57-0 96%
Reaction Conditions:
with lithium hexamethyldisilazane in tetrahydrofuran at 75; for 1 h;Flow reactor;
Steps:
Synthesis of benzoylacetonitrile derivatives in Scheme 2
General procedure: Syringe A (50 mL) was filled with a solution that N,N-dimethylbenzamides (9.0 mmol) was dissolved in 45.0 mL THF. And syringe B (50 mL) was filled with a solution that acetonitrile (18.0 mmol) and LiHMDS (27.0 mmol) were dissolved in 45.0 mL THF. The solution was injected into the tube using a syringe pump (Harvard apparatus 70-3311). Swagelok tee union was used as the connector. The two solutions are mixed as they pass through the tee union. Reaction proceeded as the solution flowed through the PTFE tube (O.D.: 1/16 in., I.D.: 1/32 in.) at 75 °C. The tube was coiled for effective mixing. Back pressure regulator (40 psi, Upchurch scientific U-605) was added to the system to suppress vaporization of the solution. The reaction mixture was collected for 1 h after a sufficient time for the system to reach the steady-state. The collected reaction mixture was quenched with water and extracted with EtOAc. The organic layer was dried over MgSO4 and removed by evaporator. The resulting reaction mixture was purified by silica gel column chromatography (eluent: Hexane/EtOAc = 10/1).
References:
Kim, Joonyoung;Park, Myeong Seong;Lee, Sunwoo;Song, Kwang Ho [Tetrahedron Letters,2022,vol. 111,art. no. 154201]
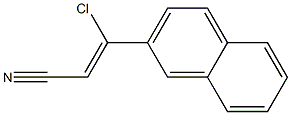
78831-76-2
0 suppliers
inquiry

613-57-0
28 suppliers
inquiry