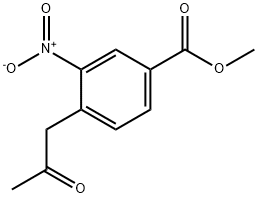
3-Nitro-4-(2-oxo-propyl)-benzoic acid methyl ester synthesis
- Product Name:3-Nitro-4-(2-oxo-propyl)-benzoic acid methyl ester
- CAS Number:206066-05-9
- Molecular formula:C11H11NO5
- Molecular Weight:237.21
Yield:206066-05-9 589 mg
Reaction Conditions:
in N,N-dimethyl-formamide at 160; for 0.333333 h;Microwave irradiation;
Steps:
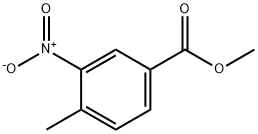
69.2 (2) Synthesis of methyl 3-nitro-4-(2-oxopropyl)benzoate [69-2] (hereinafter referred to as a compound [69-2])
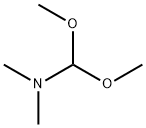
To a solution of the compound [69-1] obtained in the process (1) (693 mg) inN,N-dimethylformamide (3.5 mL) was added N,N-dimethylformamide dimethyl acetal (1.5 mL) at room temperature, and then the reaction mixture was subjected to microwave irradiation at 160°C for 20 minutes. The reaction mixture was concentrated under reduced pressure to give a reddish brown solid. To a solution of the obtained solid in chloroform (6 mL)were added pyridine (0.46 mL) and acetyl chloride (0.36 mL) at room temperature, and then the reaction mixture was stirred at room temperature for 3 days. The reaction mixture was quenched with water, and concentrated under reduced pressure. To the obtained residue were added 1,4-dioxane (3 mL) and water (1.5 mL), and then the reaction mixture was stirred at 100°C for 18 hours. The reaction mixture was concentrated under reduced pressure, and then quenched with water, and extracted with ethyl acetate. The obtained organic layer was dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography to give the titled compound (589 mg) as a brown oil. 1H-NMR (400 MHz, CDCl3) δ: 8.75 (1H, d, J = 1.2Hz), 8.24 (1H, dd, J = 7.8, 2.0 Hz), 7.38 (1H, d, J = 7.8Hz), 4.20 (2H, s), 3.97 (3H, s), 2.35 (3H, s).
References:
EP2669270,2013,A1 Location in patent:Paragraph 0525-0526

96-98-0
359 suppliers
$8.00/10g

206066-05-9
2 suppliers
inquiry