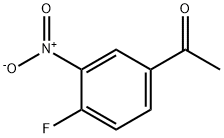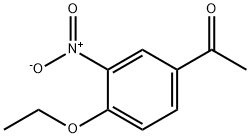
3-nitro-4-ethoxyacetophenone synthesis
- Product Name:3-nitro-4-ethoxyacetophenone
- CAS Number:24430-26-0
- Molecular formula:C10H11NO4
- Molecular Weight:209.2
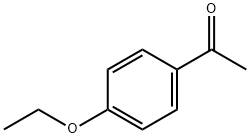
1676-63-7
260 suppliers
$6.00/1g

24430-26-0
17 suppliers
inquiry
Yield:24430-26-0 58%
Reaction Conditions:
with nitric acid;acetic anhydride at 0; for 1 h;
Steps:
7
In to a round bottom flask was placed 4-ethoxyacetophenone (5.31 g, 32.3 mmol) in acetic anhydride (11 mL). 80% fuming HNO3 (7.9 mL) was added drop wise at 000. After stirring for 1 h, the reaction mixture was poured into ice water (250 mL). The organic compound was extracted with EtOAc (2 x 100 mL). The combined organic layer was washed with brine and dried over MgSO4, filtrated and concentrated under reduce pressure. The residue was purified by flash column chromatography on silica gel (cyclohexane/EtOAc: 9/1) to afford the desired product(3.95 g, 58%).1H NMR (300 MHz, CDCI3): 6 [ppm] = 8.4 (d, J= 2.1 Hz, 1H), 8.12 (dd, J= 8.7 and2.3 Hz, 2H) 7.13 (d, J = 8.9 Hz, 2H), 4.27 (q, J = 7.0 Hz, 2H), 2.59 (5, 3H), 1.50 (t, J = 7.0 Hz, 3H).
References:
WO2018/178277,2018,A1 Location in patent:Paragraph 00144
99-93-4
833 suppliers
$5.00/25g

24430-26-0
17 suppliers
inquiry