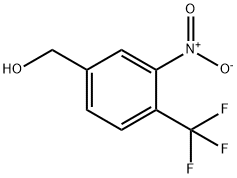
3-NITRO-4-(TRIFLUOROMETHYL)BENZYL ALCOHOL synthesis
- Product Name:3-NITRO-4-(TRIFLUOROMETHYL)BENZYL ALCOHOL
- CAS Number:372119-86-3
- Molecular formula:C8H6F3NO3
- Molecular Weight:221.13
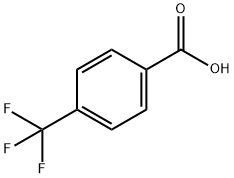
455-24-3
365 suppliers
$5.00/5g

372119-86-3
14 suppliers
inquiry
Yield:372119-86-3 20%
Reaction Conditions:
with hydrogenchloride;nitric acid in tetrahydrofuran;methanol;sulfuric acid;
Steps:
4.A [3-nitro-4-(trifluoromethyl)phenyl]methanol
Example 4A [3-nitro-4-(trifluoromethyl)phenyl]methanol 4-(Trifluoromethyl)benzoic acid (15 g, 78.9 mmol) in 90 mL of concentrated sulfuric acid was treated dropwise with a mixture of fuming nitric acid (2 mL) and sulfuric acid (24 mL). The reaction mixture was stirred at ambient temperature for 48 hours and quenched into ice-water. The resulting precipitate (12 g) was collected by filtration, washed with water and dried under reduced pressure. The resulting dry solid (12 g) was dissolved in THF (300 mL), cooled to 0° C., treated with 1M borane-tetrahydrofuran complex (80 mL) and stirred at ambient temperature overnight. Methanol (5 mL) was added dropwise followed by dropwise addition of concentrated HCl (5 mL), refluxed for 1 hour and evaporated to dryness and partitioned between water and diethyl ether. The organic layer was dried over MgSO4 and concentrated under reduced pressure. The residue was chromatographed on silica gel eluding with 30% ethyl acetate hexanes to provide the title compound (3.6 g, 20% yield). 1H-NMR (CDCl3) δ4.9 (s, 2H), 7.7 (d, 1H), 7.8 (d, 1H), 7.9 (s, 1H).
References:
US2003/65182,2003,A1
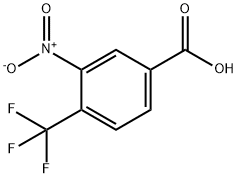
116965-16-3
117 suppliers
$7.00/250mg

372119-86-3
14 suppliers
inquiry