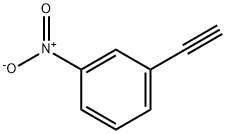
3-NITROPHENYLACETYLENE synthesis
- Product Name:3-NITROPHENYLACETYLENE
- CAS Number:3034-94-4
- Molecular formula:C8H5NO2
- Molecular Weight:147.13
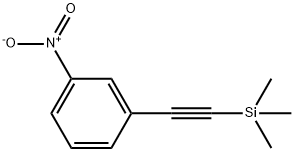
183322-33-0
3 suppliers
inquiry

3034-94-4
139 suppliers
$9.00/250mg
Yield: 100%
Reaction Conditions:
with tetrabutyl ammonium fluoride in tetrahydrofuran at 20; for 2 h;
Steps:
1-ethynyl-3-nitrobenzene (41)
To a solution of trimethyl((3-nitrophenyl)ethynyl)silane (4.24 g, 19.34 mmol) in THF (39 mL) was added tetrabutylammonium fluoride solution (21.27 ml, 1M in THF). After addition, the reaction was stirred at ambient temperature for 2 h. All the volatile solvent was removed under reduced pressure, and the residue was dissolved in DCM (20 ml). Water (15 mL) was added, and the aqueous layer was extracted with DCM (15 mL X 2). The combined organic layer was washed with brine (30 mL), dried over Na2SO4, and filtered. The organic layer was concentrated under reduced pressure and the residue was purified by silica gel chromatography, by column chromatography with hexane/ethyl acetate (20/1, v/v) to obtain the compound 41 as a yellow solid (1.77 mg, quant. yield): 1H NMR (400 MHz, CDCl3) _ 8.31 (t, J = 1.9 Hz, 1H), 8.20 (dd, J = 8.3, 1.1 Hz, 1H), 7.79 (dt, J = 7.7, 1.3 Hz, 1H), 7.53 (t, J = 8.0 Hz, 1H), 3.24 (s, 1H); 13C NMR (100 MHz, CDCl3) _ 137.9, 129.5, 127.0, 124.0, 123.6, 81.2, 80.0.
References:
BOARD OF REGENTS, THE UNIVERSITY OF TEXAS SYSTEM;DALBY, Kevin N.;EDUPUGANTI, Ramakrishna;TALIAFERRO, Juliana;LEE, Juhyeon WO2018/160967, 2018, A1 Location in patent:Page/Page column 81
99419-61-1
0 suppliers
inquiry

3034-94-4
139 suppliers
$9.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

3034-94-4
139 suppliers
$9.00/250mg
99-61-6
654 suppliers
$5.00/10g

3034-94-4
139 suppliers
$9.00/250mg
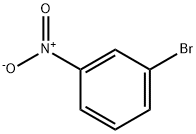
585-79-5
10 suppliers
$15.00/1g

3034-94-4
139 suppliers
$9.00/250mg