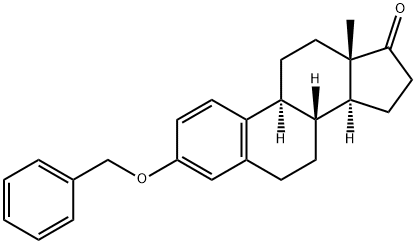
3-O-Benzyl Estrone synthesis
- Product Name:3-O-Benzyl Estrone
- CAS Number:858-98-0
- Molecular formula:C25H28O2
- Molecular Weight:360.49
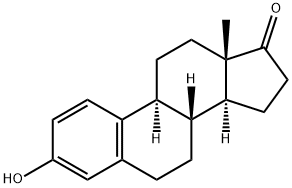
53-16-7
553 suppliers
$10.00/1g

100-39-0
424 suppliers
$10.00/10g

858-98-0
19 suppliers
$120.00/50mg
Yield:858-98-0 99%
Reaction Conditions:
with sodium hydroxide;tetra(n-butyl)ammonium hydrogensulfate in dichloromethane;water; for 24 h;Heating / reflux;
Steps:
1.A
To a solution of estrone 1 (1.00 g, 3.70 mmol) in dichloromethane (10 mL), was added benzylbromide (0.53 mL, 4.44 mmol), tetrabutylammonium hydrogen sulfate (100 mg), and a solution of sodium hydroxide (10% w/v, 5 mL). The reaction mixture was stirred vigorously at reflux for 24 h. Then, the mixture was diluted with diethyl ether (30 mL) and water (30 mL) and washed with water (4×75 mL). The organic phase was dried with magnesium sulfate, filtered and evaporated to yield a solid compound. The residue was triturated with hexanes to give a white solid in 99% yield. [0189] IR (KBr, νmax, cm-1): 1731 (CO), 1614 (CC), 1230 and 1008 (C-O). [0190] 1H-NMR (CDCl3, δ ppm): 7.41 (2H, d, J=7.6 Hz, a-CH), 7.36 (2H, t, J=7.5 Hz, b-CH), 7.29 (1H, t, J=7.2 Hz, c-CH), 7.18 (1H, d, J=8.6 Hz, 1-CH), 6.78 (1H, dd, J=2.5 Hz and J=8.5 Hz, 2-CH), 6.71 (1H, d, J=2.1 Hz, 4-CH), 5.01 (2H, s, CH2Ph), 2.88 (2H, m, 6-CH2), 2.50-1.39 (13H, No.m, 3×CH and 5×CH2), 0.89 (3H, s, 18-CH3). [0191] 13C-NMR (CDCl3, δ ppm): 220.5 (17-C), 156.8 (3-C), 137.7 (CCH2O), 137.2 (5-C), 132.2 (10-C), 128.4 (b-C), 127.7 (c-C), 127.3 (a-C), 126.2 (1-C), 114.8 (4-C), 112.3 (2-C), 69.8 (CH2Ph), 50.3 47.9, 43.9, 38.3, 35.8, 31.5, 29.6, 26.5, 25.8, 21.5, 13.8 (C-18). [0192] MS (m/e): 360 (M+), 269 (M+-C7H7). [0193] Exact mass: calculated for C25H28O2=360.2089; found=360.2095.
References:
US2004/6051,2004,A1 Location in patent:Page/Page column 7-8

100-39-0
424 suppliers
$10.00/10g

858-98-0
19 suppliers
$120.00/50mg
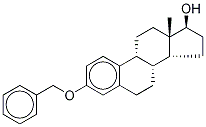
14982-15-1
8 suppliers
$139.80/10mg

858-98-0
19 suppliers
$120.00/50mg

53-16-7
553 suppliers
$10.00/1g

100-44-7
630 suppliers
$13.50/250G

858-98-0
19 suppliers
$120.00/50mg

50-28-2
647 suppliers
$15.00/1g

858-98-0
19 suppliers
$120.00/50mg