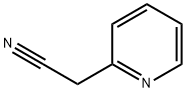
3-oxo-2-phenylbutanenitrile synthesis
- Product Name:3-oxo-2-phenylbutanenitrile
- CAS Number:57115-24-9
- Molecular formula:C9H8N2O
- Molecular Weight:160.17
Yield:57115-24-9 29%
Reaction Conditions:
with sodium ethanolate in tetrahydrofuran at 10; for 16 h;
Steps:
11.a Step a: Synthesis of 3-oxo-2-(2-pyridyl)butanenitrile
[507] To a solution of 2-(2-pyridyl)acetonitrile (2,00 g, 16,93 mmol, 1.83 mL, 1 ,00 eg) in THF (5 mL) was added EtONa (3.46 g, 50.79 mmol, 3.00 eg) and formyl chloride (2.18 g, 33.86 mmol, 2.00 eg). The reaction mixture was stirred at 10 °C for 16 h. TLC (ethyl acetate) indicated -40% of SM remained and one major new spot formed. The reaction mixture was diluted with water (50 mL), and adjusted to pH=5 with sat. aq. KHSO4 solution. Then the resulting mixture was extracted with ethyl acetate (50 mL χ 3). The organic layers were combined, dried over anhydrous NazSC , filtered and concentrated under reduced pressure to give a residue, which was purified by column chromatography on silica gel (5- 50% ethyl acetate/petroleum ether) to afford 3-oxo-2-(2-pyridyl)butanenitrile (780 mg, 4.87 mmol, 29% yield) as a yellow solid. LC-MS (ESI): m/z 161.0 { .M 1 I s ' .
References:
WO2017/210678,2017,A1 Location in patent:Paragraph 506; 507