
3-phenanthreneboronic acid synthesis
- Product Name:3-phenanthreneboronic acid
- CAS Number:1188094-46-3
- Molecular formula:C14H11BO2
- Molecular Weight:222.05

5419-55-6
372 suppliers
$12.00/5g
715-50-4
144 suppliers
$21.00/250mg

1188094-46-3
18 suppliers
inquiry
Yield: 80%
Reaction Conditions:
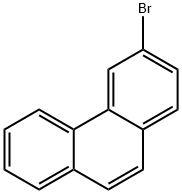
Stage #1:3-bromophenanthrene with n-butyllithium in tetrahydrofuran;hexane at -78; for 2.8 h;
Stage #2:Triisopropyl borate in tetrahydrofuran;hexane at -78 - 20; for 18 h;
Stage #3: with ammonium chloride in tetrahydrofuran;hexane
Steps:
3.d 5-(10-Phenanthren-3-ylanthracen-9-yl)-2-pyridin-2-ylpyrimidine
20 g (77.8 mmol) of 3-bromophenanthrene are dissolved in 400 ml of dry THF and cooled to -78° C.d)3.d10020 g (77.8 mmol) of 3-bromophenanthrene are dissolved in 400 ml of dry THF and cooled to -78° C. At this temperature, 40.5 ml (101.1 mmol/2.5 M in hexane) of n-BuLi are added over the course of about 20 min., and the mixture is subsequently stirred at -78° C. for a further 2.5 h. 28.7 ml (124.4 mmol) of triisopropyl borate are added as rapidly as possible at this temperature, and the reaction is slowly allowed to come to room temperature (about 18 h). 100 ml of NH4Cl solution are subsequently added to the reaction mixture, which is then extracted with ethyl acetate. The organic phase is dried over MgSO4, and the solvents are removed in vacuo. Yield: 14 g of 3-phenanthreneboronic acid, 80% of theory.
References:
Merck Patent GmbH Patents & Scientific Information;Buesing, Arne;Heil, Holger;Mujica-Fernaud, Teresa US2013/32764, 2013, A1 Location in patent:Paragraph 0106; 0107