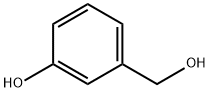
3-Phenoxybenzyl alcohol synthesis
- Product Name:3-Phenoxybenzyl alcohol
- CAS Number:13826-35-2
- Molecular formula:C13H12O2
- Molecular Weight:200.23

39515-51-0
405 suppliers
$10.00/5g

13826-35-2
307 suppliers
$6.00/5g
Yield:13826-35-2 99.2%
Reaction Conditions:
with sodium tetrahydroborate in tetrahydrofuran;1,2-dimethoxyethane for 3 h;Cooling with ice;
Steps:
1-1 Example 1-1 Preparation of the compound 3-(4-(3-phenoxybenzyloxy)phenyl)propynoic acid (HL-001)
3-Phenoxybenzaldehyde (1.20 g, 6.1 mmol) was added to a 50 mL round bottom flask.Add 6mL THF and 6mLDME dissolves it,Add NaBH4 (464 mg, 12.2 mmol) in portions under ice bath.The reaction was continued to stir for 3 hours under an ice bath.The TLC detects the tracking reaction, and after the reaction is completed, the reaction solution is slowly added with an appropriate amount of deionized water to quench it.Extracted with ethyl acetate and water,Take the organic phase and evaporate it,Purification by column chromatography gave a colorless oil.That is, 3-phenoxybenzyl alcohol (1.20 g, yield 99.2%).The obtained 3-phenoxybenzyl alcohol (450 mg, 2.3 mmol) was added to a 50 mL round bottom flask.Dissolve it in 10 mL of DCM, add PBr3 liquid (0.3 mL, 2.5 mmol) dropwise under ice bath and keep the ice bath for 3 small reactions.Time. After the reaction is completed, the excess saturated NaHCO3 solution is slowly added to the reaction solution under stirring to adjust the reaction system.It is weakly alkaline, extracted with dichloromethane and water, and the solvent of the organic phase is evaporated and purified by silica gel column chromatography to obtain colorless oil.Liquid, 3-phenoxybenzyl bromide (350 mg, yield 85.3%). The resulting 3-phenoxybenzyl bromide (350 mg, 1.9 mmol)Dissolve with 10 mL of DMF, add to a 50 mL round bottom flask, then add p-coumaric acid ethyl ester (434 mg, 2.3 mmol) andK2CO3 (1.56g, 11.3mmol), reacted at 50 ° C for 6 hours, after the reaction was completed, extracted with ethyl acetate and water, combined withAfter the organic phase was evaporated to dryness, then purified by column chromatographyYield 58.6%). The obtained white solid (410 mg, 1.1 mmol) was added to a 100 mL round bottom flask and dissolved in 20mLDCMIn the process of maintaining the ice bath, Br2 (0.2 mL, 3.9 mmol) was added dropwise and the reaction was continued for 10 min in an ice bath. TLC testAfter the completion of the reaction, Na2S2O3 (1.90 g, 12.0 mmol) and 20 mL of water were added to the reaction mixture, and stirred at room temperature for 5 min, thenThe organic phase was extracted with dichloromethane and water and evaporated to ethylamine The pale yellow solid to be obtained, KOH(280 mg, 5.0 mmol) and 15 mL of isopropanol were placed in a 100 mL round bottom flask, and the reaction was stirred at 87 ° C for 6 hours. After the reactionAfter the reaction, the reaction solution was adjusted to be acidic with a 3 mol/L aqueous solution of HCl, and then extracted with ethyl acetate and water, and the organic phase was evaporated to dryness.Purification by column chromatography gave a white solid, which gave the final product 0011 (143 mg, the total yield of the last two steps was 39.7%, purity96.5%).
References:
East China Normal University;Shanghai Bangyao Biological Technology Co., Ltd.;Zhang Hankun;Lu Weiqiang;Liu Mingyao;Hu Longlong;Jiang Xingwu;Qiu Ziliang CN109516914, 2019, A Location in patent:Paragraph 0070; 0071; 0072
15852-73-0
271 suppliers
$11.00/25g

108-95-2
727 suppliers
$14.00/25g

13826-35-2
307 suppliers
$6.00/5g
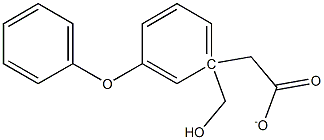
50789-44-1
0 suppliers
inquiry

13826-35-2
307 suppliers
$6.00/5g
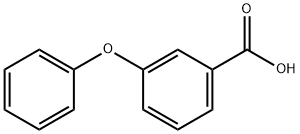
3739-38-6
195 suppliers
$6.00/1g
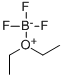
109-63-7
382 suppliers
$10.60/1gm:

13826-35-2
307 suppliers
$6.00/5g