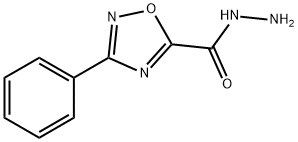
3-phenyl-1,2,4-oxadiazole-5-carbohydrazide synthesis
- Product Name:3-phenyl-1,2,4-oxadiazole-5-carbohydrazide
- CAS Number:90323-72-1
- Molecular formula:C9H8N4O2
- Molecular Weight:204.19

37760-54-6
39 suppliers
$79.75/1g

90323-72-1
9 suppliers
inquiry
Yield:90323-72-1 95%
Reaction Conditions:
with hydrazine hydrate in ethanol at 20; for 1 h;
Steps:
1 4.2.5. 3-Aryl-1,2,4-oxadiazole-5-carbohydrazides (7a-e) [17]
General procedure: In a round-bottom flask, ethyl 3-aryl-1,2,4-oxadiazole-5-carboxylate 7a-e (6mmol) was dissolved in ethanol (15mL) under magnetic stirring. Then, hydrazine hydrate (0.6mL, 12mmol) was added and a precipitate of the hydrazide readily formed. The mixture was kept under stirring at room temperature for 1h, after which the precipitated hydrazide was collected by vacuum filtration and washed with cold ethanol (50mL). The solid was transferred to another balloon and was dried under reduced pressure. 4.2.5.1 3-Phenyl-1,2,4-oxadiazole-5-carbohydrazide (7a) Yield = 1.222 g, 95% (white solid). 1H NMR (DMSO-d6, 400 MHz): δ = 10.66 (br, 1 H) 8.05 (dd, J1 = 7.9 Hz, J2 = 1.7 Hz, 2 H); 7.65-7.57 (m, 3 H); 4.99 (br, 2 H). 13C NMR (DMSO-d6, 100 MHz): δ = 169.0; 167.9; 151.7; 131.8; 129.2; 127.0; 125.5.
References:
Mayer, Jo?o C.P.;Sauer, André C.;Iglesias, Bernardo A.;Acunha, Thiago V.;Back, Davi F.;Rodrigues, Oscar E.D.;Dornelles, Luciano [Journal of Organometallic Chemistry,2017,vol. 841,p. 1 - 11]

259150-97-5
12 suppliers
$175.00/100mg

90323-72-1
9 suppliers
inquiry
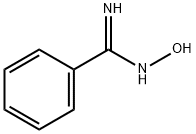
613-92-3
198 suppliers
$5.00/250mg

90323-72-1
9 suppliers
inquiry
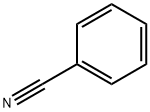
100-47-0
359 suppliers
$14.14/25gm:

90323-72-1
9 suppliers
inquiry

613-92-3
198 suppliers
$5.00/250mg

90323-72-1
9 suppliers
inquiry