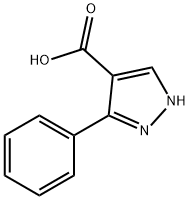
3-PHENYL-1H-PYRAZOLE-4-CARBOXYLIC ACID synthesis
- Product Name:3-PHENYL-1H-PYRAZOLE-4-CARBOXYLIC ACID
- CAS Number:5504-65-4
- Molecular formula:C10H8N2O2
- Molecular Weight:188.18
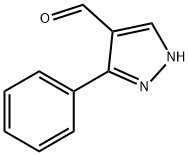
26033-20-5
168 suppliers
$10.00/1g

5504-65-4
58 suppliers
$39.50/250mg
Yield:-
Reaction Conditions:
with potassium permanganate;potassium hydroxide in 1,4-dioxane;water at 20; for 3 h;
Steps:
4.1.2.1. Procedure for preparation of compounds 13a-b, 14a-b.
General procedure: Substituted ketones (6, 5 mmol) was dissolved in ethanol (25 mL),then tert-butyl hydrazinecarboxylate (7, 5.5 mmol) and CH3COOH(0.5 mmol) were sequentially added. The solution was heated underreflux for 4 h, monitoring the progress of the reaction by TLC.After the reaction was completed, solvents were removed undervacuum. The residue was dissolved in DCM (5 mL) and CF3COOH(7.5 mmol) were added, the solution was kept stirring under rt for0.5h, after completion of the reaction, solvents were removed undervacuum, the remaining solid 8a-b was used in the next stepwithout further purification. DMF (3 mL) was slowly added phosphorusoxychloride (1 mL) under ice bath, stirring for 1 h to getVilsmeier-Haack Reagent. Then adding the 8a-b (5 mmol) to thesolution, stirring at room temperature for 30 min, then transferringto 80 C for 4 h. After the reaction was over, the resulting mixture was slowly poured into ice-cold water, a saturated solution of sodiumbicarbonate was added to neutralize the mixture, extractedwith ethyl acetate. The organic phase was dried over Na2SO4,filtered and the solvent was evaporated under reduced pressure toafford crude product 9a-b. To a stirring suspension of 9a-b(5 mmol) in 10 mL of Dioxane/water (4:1), were added of KOH(6 mmol) and potassium permanganate (6 mmol). The mixture wasstirred at room temperature for 3 h. Then slowly adding Hydrogenperoxide, MnO2 was filtered. The dioxane in the filtrate wereremoved under reduced pressure and added water (10 mL), thesolution was acidified to pH 1e2 with concentrated HCl, and thecrude product was purified by column chromatography on silica gelto afford compounds 10a-b. To a solution of 10a-b (2 mmol) indichloromethane (5 ml), EDCI (2.4 mmol) and HOBT (2.4 mmol)were added and the reaction mixture was stirred for 20 min. To thereaction mixture compound 12 (2 mmol) was added and stirredovernight at room temperature. The reaction was diluted withwater (30 mL) and extracted with DCM (2 30 mL), the organiclayers were combined, dried over MgSO4, filtered, and evaporatedunder reduced pressure. The product 13a-bwas purified by columnchromatography (EtOAc/hexane 3/1). Compounds 14a-b wereprepared through similar procedure as used for the synthesis ofcompound 13a-b except 10a-b were replaced by 11a-b.
References:
Liang, Qianqian;Liu, Hong-Min;Ma, Li-Ying;Ren, Hongmei;Wu, Yang;Zhang, Kun;Zhang, Xinhui;Zhao, Bing;Zheng, Yi-Chao [European Journal of Medicinal Chemistry,2020,vol. 192]
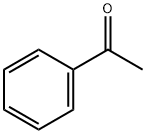
98-86-2
750 suppliers
$9.00/5g

5504-65-4
58 suppliers
$39.50/250mg

181867-24-3
22 suppliers
$45.00/10mg

5504-65-4
58 suppliers
$39.50/250mg

13466-30-3
10 suppliers
inquiry

5504-65-4
58 suppliers
$39.50/250mg
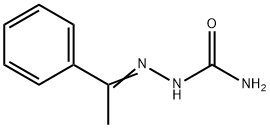
2492-30-0
18 suppliers
$60.00/500mg

5504-65-4
58 suppliers
$39.50/250mg