
3-PHENYL-2-FUROIC ACID synthesis
- Product Name:3-PHENYL-2-FUROIC ACID
- CAS Number:169772-63-8
- Molecular formula:C11H8O3
- Molecular Weight:188.18

14903-90-3
82 suppliers
$33.00/100mg

98-80-6
716 suppliers
$5.00/5g

169772-63-8
13 suppliers
$45.00/10mg
Yield:169772-63-8 70%
Reaction Conditions:
with sodium hydrogencarbonate;tetrakis(triphenylphosphine) palladium(0) in 1,2-dimethoxyethane;water; for 10 h;Reflux;
Steps:
9.1
To 3-bromo-2-furancarboxylic acid (540 mg, 2.8 mmol dissolved in DME (105 ml)) was added of (Ph3P)4Pd (936 mg, 0.81 mmol), and the resulting solution was stirred at room temperature for 15 min. The mixture was treated with 500 mg (3.0 mmol) of benzene boronic acid, an aqueous solution of 2M NaHCOs (4 mmol) and heated to reflux for 10 hours. After cooling to room temperature, the solvent was partially removed under reduced pressure, and the resulting mixture was extracted twice with diethyl ether. The water layer was acidified with 10% HCI and extracted with ethyl acetate (5 times). The combined organic layers were washed with brine, dried (Na2SO4), and evaporated under reduced pressure. The residue was crystallized from a mixture of n-hexane/ethyl acetate (about 8:2) to give the title compound. Yield: 70%.1H-NMR (CD3OD, 200 MHz) ?' 6.57 (d J= 1.8 Hz, 1 H, H-Fur), 7.16-7.28 (m, 3H, H-Ph), 741-7.49 (m, 2H, H-Ph), 7.59 (d J= 1.8 Hz, 1 H, H-Fur).
References:
WO2009/155413,2009,A1 Location in patent:Page/Page column 36
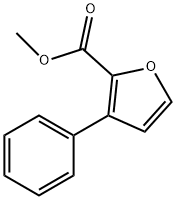
71237-44-0
4 suppliers
inquiry

169772-63-8
13 suppliers
$45.00/10mg

14903-90-3
82 suppliers
$33.00/100mg

169772-63-8
13 suppliers
$45.00/10mg
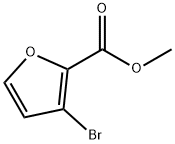
59862-77-0
12 suppliers
inquiry

169772-63-8
13 suppliers
$45.00/10mg