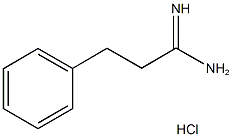
3-Phenyl-propionamidine HCl synthesis
- Product Name:3-Phenyl-propionamidine HCl
- CAS Number:24441-89-2
- Molecular formula:C9H13ClN2
- Molecular Weight:184.67

645-59-0
107 suppliers
$49.29/10g

24441-89-2
16 suppliers
inquiry
Yield:24441-89-2 11%
Reaction Conditions:
Stage #1: dihydrocinnamonitrilewith hydrogenchloride in methanol at -10 - 20; for 0.333333 h;
Stage #2: with ammonia in ethanol at 80; for 18 h;
Stage #3: with hydrogenchloride in methanol;water at 20; for 1 h;
Steps:
164 Example 164: 3-phenethylpropanimidamide hydrochloride
500mg(3 .8mmol) of 3-phenylpropanenitrile as a starting material was dissolved in 10ml of methanol and cooled to -10 ‘C. The reaction solution was bubbled and saturated by HCl gasfor 20 minutes, and then agitated at room temperature for 1 hour. The reaction solution was concentrated under vacuum, poured to seal tube and reacted with the addition of 5 ml of ammonium solution (2M ethanol) at 80 °C for 18 hours. The concentrated product was dissolved in a small amount of methanol, agitated with the addition of 12N HCl (0.28ml, 3.41 mmol) at room temperature for 1 hour, and agitated with the addition of ethylacetate 10 mlfor 30 minutes. The produced solid was filtered, washed with ethylacetate and dried under vacuum to produce the title compound in white solid (81 mg, 11 %). 1H NMR (600MHz, DMSO-D6) 6 8.14 (t, J 7.8Hz, 2H), 8.07 (d, J 7.8Hz, 2H), 7.98 (t, J= 7.8Hz, 1H), 3.77 (t, J’ 7.8Hz, 2H), 3.52 (t, J’ 7.8Hz, 2H)LCMS: 149.2 [M+H]+
References:
WO2015/160220,2015,A1 Location in patent:Page/Page column 83
103-63-9
503 suppliers
$9.00/10g

24441-89-2
16 suppliers
inquiry