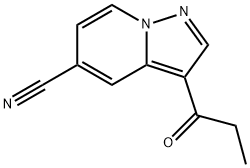
3-Propionylpyrazolo[1,5-a]pyridine-5-carbonitrile synthesis
- Product Name:3-Propionylpyrazolo[1,5-a]pyridine-5-carbonitrile
- CAS Number:1233326-34-5
- Molecular formula:C11H9N3O
- Molecular Weight:199.21
Yield:1233326-34-5 71%
Reaction Conditions:
Stage #1: pyridine-4-carbonitrilewith mesitylenesulfonylhydroxylamine in dichloromethane at 0; for 2 h;
Stage #2: 1-(trimethylsilyl)-1-pentyne-3-onewith potassium fluoride;potassium carbonate in N,N-dimethyl-formamide at 20; for 18 h;
Steps:
4.1.4. Synthesis of pyrazolo[1,5-a]pyridine-3-ketones 12b-f
General procedure: Unless otherwise stated, the following method was used. A fresh solution of MSH23 in CH2Cl2 (1 equiv) was added to the pyridine (1 equiv) in CH2Cl2 (10 mL) at 0 °C. After 2 h, the solvent was removed in vacuo. The residue was taken up in dry DMF (8 mL), then the alkynyl ketone (1 equiv) and K2CO3 (2 equiv) were added, and the suspension stirred at room temperature for 18 h. The reaction mixture was diluted with water and extracted twice with EtOAc. The combined organic phases were washed three times with water then with brine, dried (Na2SO4) and the solvent removed in vacuo. Chromatography (eluting with a hexanes: EtOAc gradient, unless otherwise stated) gave the pyrazolo[1,5-a]pyridine.
References:
Kendall, Jackie D.;O'Connor, Patrick D.;Marshall, Andrew J.;Frédérick, Rapha?l;Marshall, Elaine S.;Lill, Claire L.;Lee, Woo-Jeong;Kolekar, Sharada;Chao, Mindy;Malik, Alisha;Yu, Shuqiao;Chaussade, Claire;Buchanan, Christina;Rewcastle, Gordon W.;Baguley, Bruce C.;Flanagan, Jack U.;Jamieson, Stephen M.F.;Denny, William A.;Shepherd, Peter R. [Bioorganic and Medicinal Chemistry,2012,vol. 20,# 1,p. 69 - 85] Location in patent:experimental part

