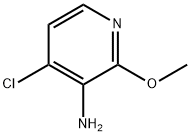
3-Pyridinamine, 4-chloro-2-methoxy- synthesis
- Product Name:3-Pyridinamine, 4-chloro-2-methoxy-
- CAS Number:934180-49-1
- Molecular formula:C6H7ClN2O
- Molecular Weight:158.59
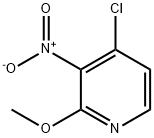
934180-48-0
32 suppliers
$45.00/10mg

934180-49-1
9 suppliers
inquiry
Yield:934180-49-1 76%
Reaction Conditions:
Stage #1: 4-chloro-2-methoxy-3-nitropyridinewith lithium hydroxide monohydrate;stannous chloride in ethyl acetate at 70; for 2 h;
Stage #2: with anhydrous sodium carbonate in lithium hydroxide monohydrate;ethyl acetate at 20; pH=9 - 10;
Steps:
b
Tin(ll)chloride dihydrate (12 g, 53.19 mmol) was added to a solution of 4-chloro-2- methoxy-3-nitropyridine (2 g, 10.64 mmol) in ethyl acetate (30 ml.) and the resultant suspension heated at 70 °C with stirring for 2 h. The reaction mixture was cooled to ambient temperature, the pH adjusted to pH 9-10 by addition of saturated sodium carbonate (aq.) and extracted with ethyl acetate (3 x 10OmL). The combined extracts were dried (MgSO4) and concentrated in vacuo to give the crude product. Purification by chromatography on silica gel with EtOAc:heptane (1 :9, v/v) as eluent afforded the product as a colourless oil (1.28 g, 76 %).Data for 3-amino-4-chloro-2-methoxypyridine: MS (ESI) m/z: 159/161 ([M+H]+).
References:
WO2007/39563,2007,A1 Location in patent:Page/Page column 24-25