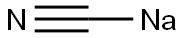
3-Pyridineacetonitrile, 6-Methoxy-2-Methyl- synthesis
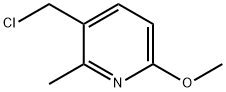
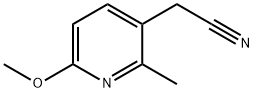
- Product Name:3-Pyridineacetonitrile, 6-Methoxy-2-Methyl-
- CAS Number:853569-73-0
- Molecular formula:C9H10N2O
- Molecular Weight:162.19
Yield:853569-73-0 91.4%
Reaction Conditions:
with sodium iodide in water;isopropyl alcohol;
Steps:
14
About half the volume of the solution prepared above as described inExample 13 was distilled off and the remainder diluted with isopropanol (7.4 L). Asolution of sodium cyanide (1.79 kg, 36.6 moles, 4.9 eq) and sodium iodide (0.11 kg, 0.73 moles, 0.1 eq) in water (4.6 L) was charged. The reaction was stirred until LC indicated a benzyl chloride/benzyl cyanide ratio of 1/100 (at) 220 nm. The layers were allowed to settle and the aqueous layer was re-extracted with ethyl acetate (4.2 L). The combined organic solutions were concentrated at 35-40 °C to brown solids. This was dissolved into water (1.5 L) and ethyl acetate (8.2 L) and the layers were separated. The aqueous layer was re-extracted with ethyl acetate (2.6 L) and the combined organic layers washed with a mixture of 1:1 saturated brine plus water (1.3 L). The organic solution was .dried with magnesium sulfate (0.26 kg), filtered, and concentrated at 25-35 °C. The volume distilled off was replaced with ethyl acetate until the water content was <400 ppm. The weight of the organic solution was 2.68 kg (41.3 wt %). This corresponds to a 91.4% solution yield.
References:
WO2005/51954,2005,A2 Location in patent:Page/Page column 41; 42