3-Pyridinecarbonitrile, 2-amino-5-chloro- synthesis
- Product Name:3-Pyridinecarbonitrile, 2-amino-5-chloro-
- CAS Number:869557-28-8
- Molecular formula:C6H4ClN3
- Molecular Weight:153.57
Yield:869557-28-8 100%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0) in 1-methyl-pyrrolidin-2-one at 110; for 5 h;Further stages;
Steps:
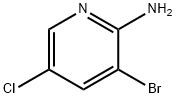
31.b Synthesis of 2-amino-5-chloronicotinonitrile
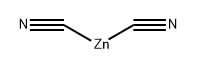
3-Bromo-5-chloropyridin-2-amine (2700.0 mg, 13.00 mmol) was dissolved in NMP (60.0 mL), and then Zn(CN)2 (2300.0 mg, 19.50 mmol) and Pd(PPh3)4 (1500.0 mg, 1.30 mmol) were added thereto. The reaction mixture was stirred at 110° C. for 5 hours and then cooled to room temperature. Water and EtOAc were added to the reaction mixture, and it was stirred for 10 minutes and then filtered through celite. The filtrate was extracted with EtOAc. The organic layer was washed with brine, dried over anhydrous Na2SO4, filtered and then distilled under reduced pressure. The residue was purified by column chromatography (EtOAc:n-Hex=1:9) on silica. The fractions containing the product were collected and evaporated to obtain white solid compound of 2-amino-5-chloronicotinonitrile (1900.0 mg, 100%). [0624] LCMS ESI(+): 154 (M+1), 156 (M+3) [0625] 1H-NMR (300 MHz, DMSO-d6); δ: 8.22 (d, 1H, J=2.7 Hz), 8.07 (d, 1H, J=2.7 Hz), 7.14 (s, 2H)
References:
US2014/315888,2014,A1 Location in patent:Paragraph 0623-0625
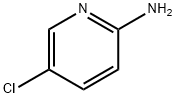
1072-98-6
696 suppliers
$5.00/1g

869557-28-8
42 suppliers
inquiry