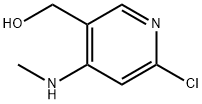
3-PyridineMethanol, 6-chloro-4-(MethylaMino)- synthesis
- Product Name:3-PyridineMethanol, 6-chloro-4-(MethylaMino)-
- CAS Number:449811-30-7
- Molecular formula:C7H9ClN2O
- Molecular Weight:172.61
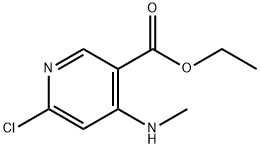
449811-28-3
21 suppliers
$298.00/500mg

449811-30-7
14 suppliers
$359.04/1g
Yield:449811-30-7 96%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 20; for 0.5 h;Inert atmosphere;
Steps:
1 (6-chloro-4-(methylamino)pyridin-3-yl)methanol (53):
The solution of 52 (100 mg, 0.46 mmol) in THF (2 mL) was added dropwise to the suspension of LAH (27 mg, 0.69 mmol) in THF (5 mL) at 0 °C and allowed to stir at room temperature for 30 min. The reaction mixture was then cooled at 0 °C and 15% NaOH (2 mL) and water (4 mL) was added dropwise. The reaction mixture was allowed to stir for 1 h, filtered and washed with EtOAc. Solvent was evaporated and the extract was purified by column chromatography using silica gel (2.5% MeOH/DCM) to afford 53 (77mg, 96%) as white solid. lH NMR (400 MHz, CD3OD) δ (ppm) 7.73 (s, 1H), 6.51 (s, 1H), 4.91 (s, 2H), 4.49 (s, 2H), 2.84 (s, 3H). 13C NMR (100 MHz, CDCb) δ (ppm) 155.72, 151.09, 145.84, 119.71, 102.79, 59.01, 28.13.
References:
WO2018/213219,2018,A1 Location in patent:Paragraph 0265-0266; 0021
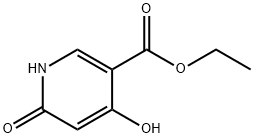
6975-44-6
202 suppliers
$17.67/250mgs:

449811-30-7
14 suppliers
$359.04/1g

105-50-0
394 suppliers
$6.00/5g

449811-30-7
14 suppliers
$359.04/1g
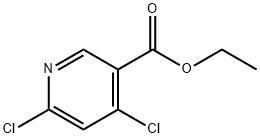
40296-46-6
323 suppliers
$6.00/1g

449811-30-7
14 suppliers
$359.04/1g