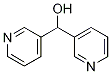
3-PyridineMethanol, alpha-3-pyridinyl- synthesis
- Product Name:3-PyridineMethanol, alpha-3-pyridinyl-
- CAS Number:89667-15-2
- Molecular formula:C11H10N2O
- Molecular Weight:186.21

35779-35-2
47 suppliers
$45.00/10mg

89667-15-2
29 suppliers
$57.00/100mg
Yield:89667-15-2 100%
Reaction Conditions:
with methanol;sodium tetrahydroborate in dichloromethane at 0; for 1 h;
Steps:
35.1
A round bottom flask was charged with di(pyridin-3-yl)methanone (500 mg, 2.72 mmol), MeOH (30 mL), and CH2C12 (15 mL) and cooled to 0 °C. NaBH4 (51 mg, 1.35 mmol) was added in one portion. The solution was stirred for 1 h at 0 °C and quenched with IN NaOH and the reaction was extracted with CH2CI2 (3X). The organic layers were combined, dried over Na2S04 and concentrated under reduced pressure. Crude di(pyridin-3-yl)methanol (505 mg, 100%) was used in the next step without further purification. XH NMR 400 MHz (CDC13) δ 8.32 (s, 2H), 8.24 (d, J= 4.8 Hz, 2H), 7.47 (d, J= 7.9 Hz, 2H), 7.09 - 7.01 (m, 2H), 5.67 (s, 1H).
References:
WO2013/103973,2013,A1 Location in patent:Paragraph 00170
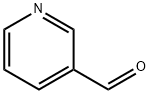
500-22-1
494 suppliers
$6.00/5g
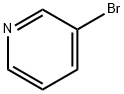
626-55-1
564 suppliers
$5.00/5/ G

89667-15-2
29 suppliers
$57.00/100mg

500-22-1
494 suppliers
$6.00/5g

626-55-1
564 suppliers
$5.00/5/ G
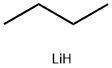
29786-93-4
0 suppliers
inquiry

89667-15-2
29 suppliers
$57.00/100mg