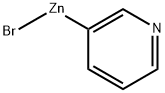
3-Pyridylzinc bromide, 0.50 M in THF synthesis
- Product Name:3-Pyridylzinc bromide, 0.50 M in THF
- CAS Number:220565-63-9
- Molecular formula:C5H4BrNZn
- Molecular Weight:223.38
Yield:220565-63-9 90 %Chromat.
Reaction Conditions:
with [CoBr2(N-(1-(pyridin-2-yl)ethylidene)quinolin-8-amine)];3-chloroprop-1-ene;trifluoroacetic acid;zinc dibromide in acetonitrile at 50;Catalytic behavior;Solvent;
Steps:
Arylzinc Reagents
General procedure: An oven-dried flask was equipped with a stirring bar and closed with a septum. Zinc dust (1.08 g, 16.5 mmol, 3.33 equiv), ZnBr2 (146 mg,0.65 mmol, 0.13 equiv) and Co(II) catalyst were added and stirred in CH3CN (4 mL). TFA (0.1 mL, 1.3 mmol, 0.26 equiv) and allyl chloride (0.17 mL, 2.05 mmol, 0.4 equiv) were added subsequently which caused a rise in temperature and gas evolution. Pyridine was added if necessary. Once the mixture again reached r.t., the aryl halide (5.0 mmol) was added and the reaction mixture was stirred at the indicated temperature 50 °C until iodolyzed aliquots showed full conversion of the starting material (GC analysis). The yield was determined by addition of an internal standard (n-decane or n-dodecane, 100 μL).
References:
Linke, Stephanie;Manolikakès, Sofia M.;Auffrant, Audrey;Gosmini, Corinne [Synthesis,2018,vol. 50,# 13,p. 2595 - 2600]
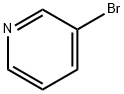
626-55-1
541 suppliers
$5.00/5/ G

220565-63-9
7 suppliers
inquiry

626-55-1
541 suppliers
$5.00/5/ G
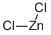
7646-85-7
532 suppliers
$10.00/25ml

220565-63-9
7 suppliers
inquiry