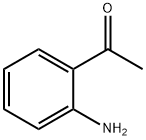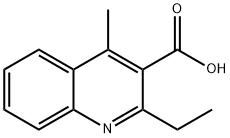
3-Quinolinecarboxylic acid, 2-ethyl-4-methyl- synthesis
- Product Name:3-Quinolinecarboxylic acid, 2-ethyl-4-methyl-
- CAS Number:847837-40-5
- Molecular formula:C13H13NO2
- Molecular Weight:215.25
Yield:847837-40-5 82%
Reaction Conditions:
in 5,5-dimethyl-1,3-cyclohexadiene at 140; for 24 h;Inert atmosphere;Friedlaender Quinoline Synthesis;
Steps:
Representative procedure for theFriedl?nder synthesis. 2-Methylquinoline-3-carboxylicacid (3a).
General procedure: To a stirred solution of 2-aminobenzaldehyde (1a, 206 mg, 1.70 mmol) in 15 mL of toluene, was added methyl acetoacetate (2a, 217 mg, 1.87 mmol, 1.1 equiv) and the solution was refluxed for 24 h during which time a light tan precipitate formed. The reaction mixture was cooled to room temperature, evaporated to one-half its volume, and then cooled to 0 °C using an ice bath. The solid was filtered and washed with 1:1 ether:hexane to give acid 3a. The compound obtained was spectroscopically pure and no further purification was necessary. Note: The reaction was also carried out using xylene as the solvent and no change in the yield or purity was observed. Reactions run in benzene, however, gave mixtures of the acid and its corresponding ester.
References:
Nammalwar, Baskar;Murie, Maeghan;Fortenberry, Chelsea;Bunce, Richard A. [Tetrahedron Letters,2014,vol. 55,# 20,p. 3181 - 3183] Location in patent:supporting information