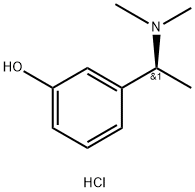
3-((S)-1-DiMethylaMino-ethyl)phenol hydrochloride synthesis
- Product Name:3-((S)-1-DiMethylaMino-ethyl)phenol hydrochloride
- CAS Number:894079-56-2
- Molecular formula:C10H16ClNO
- Molecular Weight:201.69
![3-[(1S)-1-(METHYLAMINO)ETHYL]PHENOL](/CAS/20180601/GIF/894079-42-6.gif)
894079-42-6
7 suppliers
inquiry

50-00-0
846 suppliers
$10.00/25g

64-18-6
1061 suppliers
$22.12/250ML

894079-56-2
26 suppliers
inquiry
Yield:894079-56-2 80%
Reaction Conditions:
Stage #1: 3-[(S)-1-(methylamino)ethyl]phenol;formaldehyd;formic acid for 3 h;Heating / reflux;
Stage #2: with hydrogenchloride in formic acid;water at 20; for 0.5 h;
Steps:
1
[33] (S)-3-(l-monomethylaminoethyl)phenol (Formula 7, 29.2 g, 0.192 mol, 99.6% ee) EPO
References:
WO2006/68386,2006,A1 Location in patent:Page/Page column 4-5