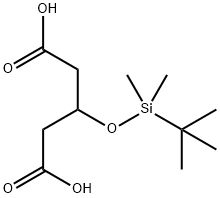
3-(tert-ButyldiMethylsilyloxy)glutaric Acid synthesis
- Product Name:3-(tert-ButyldiMethylsilyloxy)glutaric Acid
- CAS Number:113794-48-2
- Molecular formula:C11H22O5Si
- Molecular Weight:262.37
32328-03-3
190 suppliers
$15.00/1g

18162-48-6
667 suppliers
$9.00/5g

113794-48-2
9 suppliers
inquiry
Yield:113794-48-2 71%
Reaction Conditions:
Stage #1: tert-butyldimethylsilyl chloridewith 1H-imidazole in dichloromethane at 20 - 30; for 1 - 2 h;
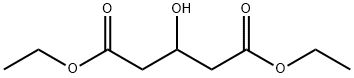
Stage #2: diethyl 3-hydroxyglutarate in dichloromethane at 20 - 30; for 4 - 6 h;
Stage #3: with hydrogenchloride;sodium hydroxide;watermore than 3 stages;
Steps:
General Preparation of 3-hvdroxy protected glutaric acid. A four-neck round bottom flask fitted with a mechanical stirrer, condenser and charging tube, was charged with methylene dichloride (675 ml) followed by charging of imidazole (187.2 g), t-butyldimethylsilyl chloride (248.3 g) under nitrogen atmosphere. Reaction mass was maintained for 1-2 hours at 20-300C followed by addition of a solution of 3-hydroxy diethyl glutarate in methylene chloride (225 g). The mass was maintained for 4-6 hours followed by water and brine washing of the reaction mass. Methylene dichloride under vacuum at 30- 350C was distilled out and residue was charged into a solution of 30-40% aq. methyl alcohol (1850 ml), sodium hydroxide (96.8 g) at 25-350C and mixed for 20-30 hours. Solvent is distilled out under vacuum at 40-450C, mass was further diluted with water and 1-12N hydrochloric acid was added to bring pH to 2.5-4 and the product was extracted with t-butyl methyl ether and concentrated to give 71 % of 3-hydroxy protected glutaric acid.
References:
WO2008/130638,2008,A2 Location in patent:Page/Page column 30
![3-[[(1,1-Dimethylethyl)dimethylsilyl]oxy]pentanedioic Acid Diethyl Ester](/CAS/GIF/91424-39-4.gif)
91424-39-4
36 suppliers
$22.00/250mg

113794-48-2
9 suppliers
inquiry

32328-03-3
190 suppliers
$15.00/1g

113794-48-2
9 suppliers
inquiry

18162-48-6
667 suppliers
$9.00/5g

113794-48-2
9 suppliers
inquiry