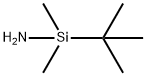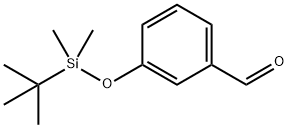
3-tert-Butyldimethylsilyloxybenzaldehyde synthesis
- Product Name:3-tert-Butyldimethylsilyloxybenzaldehyde
- CAS Number:96013-95-5
- Molecular formula:C13H20O2Si
- Molecular Weight:236.38
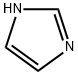
288-32-4
979 suppliers
$5.00/25g

18162-48-6
658 suppliers
$9.00/5g
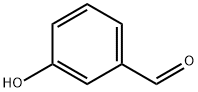
100-83-4
622 suppliers
$5.00/10g

96013-95-5
11 suppliers
inquiry
Yield:96013-95-5 126.6 g (94%)
Reaction Conditions:
in N-methyl-acetamide;
Steps:
1 (+)-3-((αR)-α-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-hydroxybenzyl)-N,N-diethylbenzamide
3-Hydroxybenzaldehyde (70 g, 0.57 mol), tert-butyldimethylsilyl chloride (92 g, 0.61 mol), and imidazole (92 g, 1.35 mol) were combined in dimethylformamide (250 mL). The mixture was stirred at room temperature, under nitrogen, for 1 hour. The solution was poured into water (1.5 L) and extracted with 2*500 mL petroleum ether (35°-60° C. boiling range). The organic solution was washed with saturated sodium chloride solution (100 mL), dried over magnesium sulfate, treated with silica gel (20 g), filtered, and concentrated in vacuo. The residue was dried further under high vacuum to yield 126.6 g (94%) of the air and light-sensitive 3-((tert-butyldimethylsilyl)oxy)benzaldehyde as an amber oil. NMR (300 MHz, CDCl3): δ0.22 (s, 6H); 0.99 (s, 9H); 7.10 (ddd, J1 =1.2 Hz, J2 =2.5 Hz, J3 =7.9 Hz, 1H); 7.32 (dd, J1 =1.5 Hz, J2 =2.4 Hz, 1H); 7.39 (t, J=7.8 Hz, 1H); 7.47 (ddd, J1 =1.3 Hz, J2 =1.3 Hz, J3 =7.6 Hz, 1H); 9.95 (s, 1H). Mass spectrum (CI--CH4) m/e: 237 (M+1, 100%). Calculated for C13 H20 O2 Si: C, 66.05; H, 8.53. Found: C, 65.95; H, 8.56.
References:
US5552404,1996,A
![Benzene, 1-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-3-[[[(1,1-dimethylethyl)dimethylsilyl]oxy]methyl]-](/CAS/20210305/GIF/96013-69-3.gif)
96013-69-3
0 suppliers
inquiry

96013-95-5
11 suppliers
inquiry

18162-48-6
658 suppliers
$9.00/5g

96013-95-5
11 suppliers
inquiry