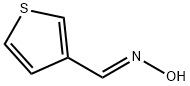
3-Thiophenecarboxaldehyde,oxime,(E)-(9CI) synthesis
- Product Name:3-Thiophenecarboxaldehyde,oxime,(E)-(9CI)
- CAS Number:148134-23-0
- Molecular formula:C5H5NOS
- Molecular Weight:127.16
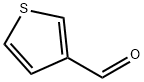
498-62-4
394 suppliers
$5.00/1g

148134-23-0
2 suppliers
inquiry
Yield:148134-23-0 99%
Reaction Conditions:
with hydroxylamine hydrochloride in pyridine;water;ethyl acetate;
Steps:
2.A Synthesis of thiophene-3-carbaldehyde oxime (Scheme V, Compound 11)
Synthesis of thiophene-3-carbaldehyde oxime (Scheme V, Compound 11) Dissolve hydroxylamine hydrochloride (725 g, 10.43 mol) in pyridine (3 L), which causes an exotherm (23-30°C). Cool (25°C) the reaction mixture, under nitrogen and add 3-thiophenecarboxaldehyde (585 g, 5.2 mol) over ~1 hour while maintaining the temperature from 22-27°C. Concentrate (60°C/50 torr) the reaction mixture to an oily residue and partition between ethyl acetate (2 L) and water (1 L). Extract the organic layer with 1 N HCI (2 x 600 mL), followed by water (3 x 500 mL). Dry (MgSO4), filter, wash the solid with ethyl acetate (500 mL) and concentrate (60 °C/60 torr) to give the title compound as a semi-solid residue (575 g, 99% Yield: 1H NMR (CDCl3, 200MHz) δ 7.3-7.55 (m, 3H), 8.2 (s, 1H), 8.7-9.2 (br s, 1H).
References:
EP1216244,2003,B1