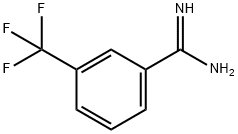
3-TRIFLUOROMETHYLBENZAMIDINE synthesis
- Product Name:3-TRIFLUOROMETHYLBENZAMIDINE
- CAS Number:26130-45-0
- Molecular formula:C8H7F3N2
- Molecular Weight:188.15
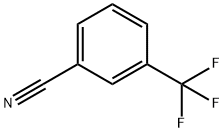
368-77-4
274 suppliers
$10.00/10g

26130-45-0
16 suppliers
inquiry
Yield:26130-45-0 100%
Reaction Conditions:
with lithium hexamethyldisilazane in tetrahydrofuran;toluene at 20;Inert atmosphere;
Steps:
5.1.91. 3-(Trifluoromethyl)benzene-1-carboximidamide (46)
A microvawe vial (ovendried and under argon) was chargedwith 3-cyanobenzotrifluoride 45 (117.2 mL, 0.88 mmol, 1.0 equiv.)and anhydrous THF (2.2 mL). A solution of LiHMDS 1M in toluene(1.32 mL, 1.32 mmol, 1.5 equiv.) was added dropwise and theresulting mixture was stirred overnight at rt. The solution wasacidified until pH 1 using a solution of HCl 1N and thenconcentrated under vacuum. The residuewas basified until pH 12using a solution of NaOH. The aqueous phase was extracted 3 timeswith DCM. The organic layers were combined, washed with brine,dried over Na2SO4, filtered and concentrated, to yield 46 as an orangeoil (168 mg, 0.88 mmol, 100%). 1H NMR (400 MHz, CDCl3) δ ppm 5.19 (s, 3H), 7.55 (t, 1H, J 7.8 Hz), 7.71 (d, 1H, J 7.8 Hz), 7.80(d, 1H, J 7.8 Hz), 7.87 (s, 1H); 13C NMR (101 MHz, CDCl3) δ ppm123.1 (q, J 3.7 Hz), 125.0, 127.1 (q, J 3.7 Hz), 129.4, 129.5, 137.3,164.2.
References:
Bollenbach, Maud;Lugnier, Claire;Kremer, Mélanie;Salvat, Eric;Megat, Salim;Bihel, Frédéric;Bourguignon, Jean-Jacques;Barrot, Michel;Schmitt, Martine [European Journal of Medicinal Chemistry,2019,vol. 177,p. 269 - 290] Location in patent:supporting information
![3H-Diazirine, 3-bromo-3-[3-(trifluoromethyl)phenyl]-](/CAS/20210305/GIF/125330-15-6.gif)
125330-15-6
0 suppliers
inquiry
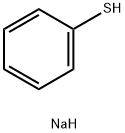
930-69-8
2 suppliers
$45.00/500mg

368-77-4
274 suppliers
$10.00/10g

26130-45-0
16 suppliers
inquiry
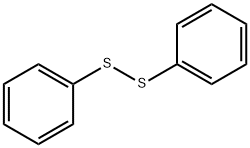
882-33-7
311 suppliers
$10.00/5g