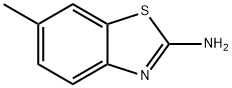
2-Thiophenecarboxamide,N-(6-methyl-2-benzothiazolyl)- synthesis
- Product Name:2-Thiophenecarboxamide,N-(6-methyl-2-benzothiazolyl)-
- CAS Number:301236-19-1
- Molecular formula:C13H10N2OS2
- Molecular Weight:274.36
Yield:301236-19-1 23%
Reaction Conditions:
in dichloromethane at 20 - 70; for 5 h;Alkaline conditions;
Steps:
3.3. General procedure for target compounds 1-28
General procedure: Compounds H-R1 (0.01 mol) or NH2-R2-NH2 (0.005 mol) were completely dissolved inCH2Cl2.Triethylamine (Et3N) or pyridine (3 mL) was added to the solution (Figure 2). Understirring, the carboxylic acid chloride (1a or 2a) was added drop by drop to the mixture at roomtemperature. Afterwards, the reaction mixture was further stirred for 5 h at 20-70°C. Thereaction was monitored by periodic TLC. After the completion of the reaction, the reactionmixture was washed with HCl (10%) and NaOH (10%) in turn. The solvent was removed underreduced pressure. Compounds 9, 15 and 17 were obtained pure directly by this method. Thecrude products of compounds 23-28 were re-crystallised with the mixture of DMSO and water(15:1), and the rest were re-crystallised with anhydrous ethanol. The purity of the synthesisedcompounds was checked by TLC.
References:
Wang, Xuesong;Gao, Sumei;Yang, Jian;Gao, Yang;Wang, Ling;Tang, Xiaorong [Natural Product Research,2016,vol. 30,# 6,p. 682 - 688]
527-72-0
480 suppliers
$5.00/10g

301236-19-1
10 suppliers
inquiry