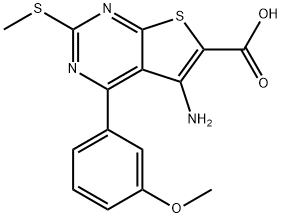
THIENO[2,3-D]PYRIMIDINE-6-CARBOXYLIC ACID,5-AMINO-4-(3-METHOXYPHENYL)-2-(METHYLTHIO)-(WXG01920) synthesis
- Product Name:THIENO[2,3-D]PYRIMIDINE-6-CARBOXYLIC ACID,5-AMINO-4-(3-METHOXYPHENYL)-2-(METHYLTHIO)-(WXG01920)
- CAS Number:301847-46-1
- Molecular formula:C15H13N3O3S2
- Molecular Weight:347.41
![THIENO[2,3-D]PYRIMIDINE-6-CARBOXYLIC ACID,5-AMINO-4-(3-METHOXYPHENYL)-2-(METHYLTHIO)-,ETHYL ESTER(WXG01919)](/CAS/20180703/GIF/301846-06-0.gif)
301846-06-0
6 suppliers
inquiry
![THIENO[2,3-D]PYRIMIDINE-6-CARBOXYLIC ACID,5-AMINO-4-(3-METHOXYPHENYL)-2-(METHYLTHIO)-(WXG01920)](/CAS/20180629/GIF/301847-46-1.gif)
301847-46-1
5 suppliers
inquiry
Yield:-
Reaction Conditions:
with lithium hydroxide in 1,4-dioxane;water at 80; for 48 h;
Steps:
1.d (d). 5-Amino-4-(3-methoxyphenyl-2-methylthio-thieno[2,3-d]pyrimidine-6-carboxylic acid
Ethyl 5-amino-4-(3-methoxyphenyl)-2-methylthio-thieno[2,3-d]pyrimidine-6-carboxylate (example 1c, 9.27 g) was dissolved in a mixture of 1,4-dioxane (270 ml) and H2O (30 ml). Lithium hydroxide (10 g) was added and the mixture was stirred at 80° C. for 48 h. 1,4-Dioxane was removed from the mixture by evaporation and the residue was taken up in H2O. The remaining solution was acidified to pH 2 by adding aq. 3N aq. HCl. The resulting precipitate was filtered off and washed with H2O. Traces of water in the precipitate were removed by coevaporation with 1,4-dioxane and then with diethylether and drying in vacuo at 50° C. overnight. Yield: 8.45 g MS-ESI: [M + H]+ = 348.0 TLC: Rf = 0.2, silica gel, CH2Cl2/CH3OH = 9/1 (v/v).
References:
US2003/225113,2003,A1 Location in patent:Page 12

128640-73-3
4 suppliers
inquiry
![THIENO[2,3-D]PYRIMIDINE-6-CARBOXYLIC ACID,5-AMINO-4-(3-METHOXYPHENYL)-2-(METHYLTHIO)-(WXG01920)](/CAS/20180629/GIF/301847-46-1.gif)
301847-46-1
5 suppliers
inquiry
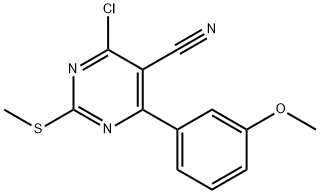
128666-82-0
6 suppliers
inquiry
![THIENO[2,3-D]PYRIMIDINE-6-CARBOXYLIC ACID,5-AMINO-4-(3-METHOXYPHENYL)-2-(METHYLTHIO)-(WXG01920)](/CAS/20180629/GIF/301847-46-1.gif)
301847-46-1
5 suppliers
inquiry
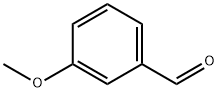
591-31-1
420 suppliers
$10.00/5g
![THIENO[2,3-D]PYRIMIDINE-6-CARBOXYLIC ACID,5-AMINO-4-(3-METHOXYPHENYL)-2-(METHYLTHIO)-(WXG01920)](/CAS/20180629/GIF/301847-46-1.gif)
301847-46-1
5 suppliers
inquiry