2-(4-CHLOROPHENYL)-6,7-DIMETHOXY-4H-3,1-BENZOXAZIN-4-ONE synthesis
- Product Name:2-(4-CHLOROPHENYL)-6,7-DIMETHOXY-4H-3,1-BENZOXAZIN-4-ONE
- CAS Number:303091-14-7
- Molecular formula:C16H12ClNO4
- Molecular Weight:317.72
Yield:-
Reaction Conditions:
with pyridine at 0 - 5;
Steps:
2-(4-Chlorophenyl)-6-methoxy-4 H -benz[ d ][1,3]oxazin-4-one (5a).
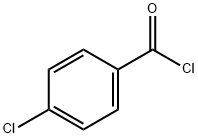
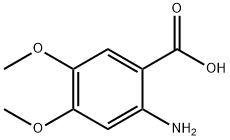
General procedure: Method A: 4-Chlorobenzoic acid (6.0 mmol) and thionyl chlo- ride (1.44 g, 0.88 ml, 12.17 mmol) were taken in a 100 ml dry RBF and refluxed for 3-4 hrs under anhydrous conditions. After com- pletion of the reaction, excess of thionyl chloride was removed un- der vacuum. 2-Amino-5-methoxybenzoic acid, 4a (1.0 g, 5.828 mM) dissolved in dry pyridine (4.6 g, 4.69 ml, 50.71 mM) was added drop wise to the above acid chloride at 0-5 °C with continuous stir- ring. After completion of the reaction, the reaction mixture was poured on crushed ice. The precipitated product was filtered out and washed with the saturated solution of NaHCO 3 . The dry solid was recrystallized from acetone to afford a pure off-white solid (5a).
References:
Khadse, Atul N.;Savsani, Hardik H.;Chikhale, Rupesh V.;Ghuge, Rahul B.;Prajapati, Dixit R.;Kureshi, Goshiya;Murumkar, Prashant R.;Patel, Kirti V.;Rajput, Sadhana J.;Yadav, Mange Ram [Journal of Molecular Structure,2022,vol. 1270,art. no. 133974]
6315-89-5
303 suppliers
$7.00/10g

303091-14-7
5 suppliers
inquiry
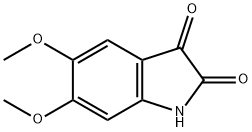
4722-81-0
11 suppliers
inquiry

303091-14-7
5 suppliers
inquiry
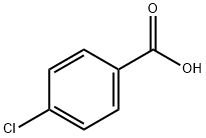
74-11-3
560 suppliers
$5.00/1g

303091-14-7
5 suppliers
inquiry