
TERT-BUTYL 4-[2-NITRO-4-(TRIFLUOROMETHYL)PHENYL]-1,4-DIAZEPANE-1-CARBOXYLAT E synthesis
- Product Name:TERT-BUTYL 4-[2-NITRO-4-(TRIFLUOROMETHYL)PHENYL]-1,4-DIAZEPANE-1-CARBOXYLAT E
- CAS Number:306934-72-5
- Molecular formula:C17H22F3N3O4
- Molecular Weight:389.37
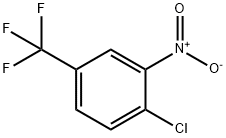
121-17-5
7 suppliers
$19.00/250g
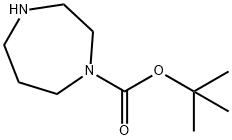
112275-50-0
243 suppliers
$9.00/1g
![TERT-BUTYL 4-[2-NITRO-4-(TRIFLUOROMETHYL)PHENYL]-1,4-DIAZEPANE-1-CARBOXYLAT E](/CAS/GIF/306934-72-5.gif)
306934-72-5
13 suppliers
$58.00/250mg
Yield:306934-72-5 97%
Reaction Conditions:
with sodium carbonate in N,N-dimethyl-formamide at 110; for 17 h;
Steps:
/e//- Butyl 4-(2-nitro-4-(trifluoromethyl)phenyl)-l,4-diazepane-l-carboxylate
A suspension of l-chloro-2-nitro-4-(trifluoromethyl)benzene (2.25 g, 9.99 mmol), tert- butyl 1,4-diazepane-l-carboxylate (2.0 g, 9.99 mmol) and solid sodium carbonate (3.18 g, 29.96 mmol) in anhydrous DMF (30 mL) was heated to 110°C for 17 h. The resulting reaction mixture was allowed to cool to room temperature and the reaction mixture was filtered through a short pad of Celite, eluting with ethyl acetate. The organic filtrate was concentrated under reduced pressure. The residue was diluted with water (100 mL) and extracted with ethyl acetate (x 3). The organic extracts were combined and dried over anhydrous Na2SC>4. The solvent was removed under reduced pressure. The resulting crude material was purified by column chromatography on silica using (90% ethyl acetate in hexane) as an eluent to afford the title product as a light yellow solid (3.78 g, 97%). NMR (400 MHz, DMSO-d6) d 1.12-1.20 (m, 9H), 1.80 (br s, 2H), 3.12-3.24 (m, 2H), 3. SO SAS (m, 2H, merged in moisture residual of DMSO), 3.50 (t, J = 6 Hz, 2H), 3.61-3.69 (m, 2H), 7.35-7.40 (m, 1H), 7.72 (d, J = 8.8 Hz, 1H), 8.02-8.03 (m, 1H); LCMS: [M+-56] 334.08 m/z, 100% purity.
References:
WO2022/13555,2022,A1 Location in patent:Page/Page column 19-20