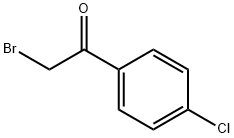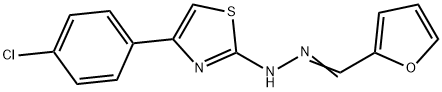
(E)-4-(4-chlorophenyl)-2-(((E)-furan-2-ylmethylene)hydrazono)-2,3-dihydrothiazole synthesis
- Product Name:(E)-4-(4-chlorophenyl)-2-(((E)-furan-2-ylmethylene)hydrazono)-2,3-dihydrothiazole
- CAS Number:307325-12-8
- Molecular formula:C14H10ClN3OS
- Molecular Weight:303.77
Yield:307325-12-8 98%
Reaction Conditions:
in ethanol at 20;
Steps:
2.2 General procedure for the synthesis of derivatives BF1-BF30
General procedure: Equimolar amounts of the prepared thiosemicarbazone (25mmol) and α,4-dichloroacetophenone both suspended in ethanol, were reacted at room temperature under magnetic stirring for 12-24h. The suspension was filtered on Gooch filter, washed with adequate solvents (diethyl ether, petroleum ether, hexane), and dried under vacuum. All products have been purified by column chromatography (SiO2, ethyl acetate/n-hexane) to give compounds BF1-BF30 in high yield.
References:
Secci, Daniela;Carradori, Simone;Bizzarri, Bruna;Bolasco, Adriana;Ballario, Paola;Patramani, Zoi;Fragapane, Paola;Vernarecci, Stefano;Canzonetta, Claudia;Filetici, Patrizia [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 5,p. 1680 - 1689]
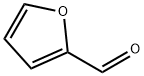
98-01-1
478 suppliers
$22.00/25g

307325-12-8
4 suppliers
inquiry
![[(E)-[(furan-2-yl)methylidene]amino]thiourea](/CAS/20200331/GIF/5419-96-5.gif)