
5-(1,3-BENZODIOXOL-5-YL)-5-METHYLIMIDAZOLIDINE-2,4-DIONE synthesis
- Product Name:5-(1,3-BENZODIOXOL-5-YL)-5-METHYLIMIDAZOLIDINE-2,4-DIONE
- CAS Number:308122-40-9
- Molecular formula:C11H10N2O4
- Molecular Weight:234.21
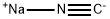
773837-37-9
0 suppliers
inquiry
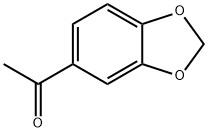
3162-29-6
209 suppliers
$18.00/5g

506-87-6
359 suppliers
$9.00/25g

308122-40-9
10 suppliers
inquiry
Yield:308122-40-9 51.2%
Reaction Conditions:
with water in ethanol at 70;
Steps:
1.1-a
Example 1; Preparation of 5-(benzo[d][1,3]dioxol-5-yl)-3-(3-(4-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-2-propylphenoxy)benzyl)-5-methylimidazolidine-2,4-dione; 1-a) Preparation of 5-(benzo[d][1,3]dioxol-5-yl)-5-methylimidazolidine-2,4-dione; 1-(Benzo[d][1,3]dioxol-5-yl)ethanone (446 mg, 2.72 mmol) was dissolved in ethanol (200 mL) and water (200 mL). The resultant mixture was added with sodium cyanide (200 mg, 4.08 mmol) and ammonium carbonate (918 mg, 9.55 mmol) and stirred at 70° C. overnight. The reaction solution was filtered, washed with water and hexane/ethyl acetate, and dried using anhydrous sodium sulfate. 5-(Benzo[d][1,3]dioxol-5-yl)-5-methylimidazolidine-2,4-dione (326 mg, yield 51.2%) was obtained as a white crystal.1H-NMR (CDCl3) δ: 1.83 (3H, s), 5.99 (2H, s), 6.81 (1H, d, J=8.3 Hz), 6.95 (1H, dd, J=2.2, 8.3 Hz), 6.99 (1H, d, J=2.2 Hz).
References:
US2010/48610,2010,A1 Location in patent:Page/Page column 17
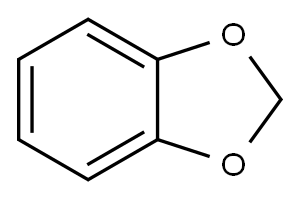
274-09-9
462 suppliers
$5.00/5g

308122-40-9
10 suppliers
inquiry