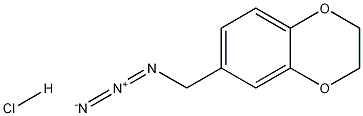
6-(azidomethyl)-2,3-dihydrobenzo[b][1,4]dioxine hydrochloride synthesis
- Product Name:6-(azidomethyl)-2,3-dihydrobenzo[b][1,4]dioxine hydrochloride
- CAS Number:31127-40-9
- Molecular formula:C9H10ClN3O2
- Molecular Weight:227.6476
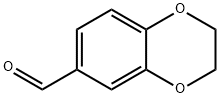
![(E)-2,3-dihydrobenzo[b][1,4]dioxine-6-carbaldehyde oxiMe](/CAS/GIF/31127-39-6.gif)
31127-39-6
43 suppliers
$14.00/100mg
![6-(azidomethyl)-2,3-dihydrobenzo[b][1,4]dioxine hydrochloride](/CAS2/GIF/31127-40-9.gif)
31127-40-9
19 suppliers
inquiry
Yield:31127-40-9 95%
Reaction Conditions:
with hydrogenchloride;hydrogen in water;isopropyl alcohol at 20; under 7600.51 Torr;Autoclave;Solvent;
Steps:
Hydrogenation of oximes 1a-k
General procedure: A solutionof oxime 1a-k (23 mmol) in isopropyl alcohol (100 mL) was loaded into the autoclave with the fl uoroplastic bush, concentratedHCl (10.5 mL) was added, and a block of the regeneratedcatalyst was fi xed. Hydrogenation was conducted for 3-5 h at20 C and a hydrogen pressure of 10 atm, which was maintainedat this level during the reaction. The reaction course was monitoredby TLC. After the initial oxime disappeared completely,the reaction solution was poured out of the autoclave, the catalystblock was washed with methanol (3×30 mL), and the combinedsolutions were fi ltered from mechanical impurities. The solventwas evaporated, and analytically pure aromatic benzylamines3a-k were obtained.When hydrogenation was conducted in methanol (100 mL),solvent removal was followed by drying residue using azeotropicdistillation with isopropyl alcohol (250 mL). For the hydrogenationof compound 1k, the reaction mixture was additionallypurifi ed by refl ux with active carbon for 1 h. The blockcatalyst was regenerated directly in the reactor at 400 C ina hydrogen fl ow and further used in the next hydrogenationprocedure.
References:
Ignatov;Varakutin;Solov’eva;Karmanova;Kozlov;Semenova;Semenov [Russian Chemical Bulletin,2018,vol. 67,# 8,p. 1394 - 1400][Izv. Akad. Nauk, Ser. Khim.,2018,# 8,p. 1394 - 1400,7]
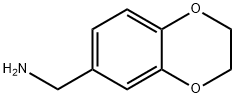
17413-10-4
98 suppliers
$45.00/50mg
![6-(azidomethyl)-2,3-dihydrobenzo[b][1,4]dioxine hydrochloride](/CAS2/GIF/31127-40-9.gif)
31127-40-9
19 suppliers
inquiry
29668-44-8
271 suppliers
$6.00/1g
![6-(azidomethyl)-2,3-dihydrobenzo[b][1,4]dioxine hydrochloride](/CAS2/GIF/31127-40-9.gif)
31127-40-9
19 suppliers
inquiry