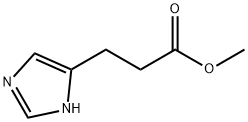
METHYL 3-(1H-IMIDAZOL-4-YL)-PROPANOATE HYDROCHLORIDE synthesis
- Product Name:METHYL 3-(1H-IMIDAZOL-4-YL)-PROPANOATE HYDROCHLORIDE
- CAS Number:31434-93-2
- Molecular formula:C7H10N2O2
- Molecular Weight:154.17

67-56-1
737 suppliers
$7.29/5ml-f

1074-59-5
95 suppliers
$45.00/50mg

31434-93-2
62 suppliers
inquiry
Yield: 90%
Reaction Conditions:
sulfuric acid for 15 h;Heating / reflux;
Steps:
38
[0380] A suspension of urocanic acid (2.00 g, 14.5 mmol) in H2O (40 mL) was shaken at room temperature with a suspension of 10% Pd/C (200 mg, 0.19 mmol) under hydrogen atmosphere (30 psi) for 2 h. The catalyst was removed by filtration, and the filtrate was concentrated in vacuo to give a colourless solid (1.95 g, 96%). 1H NMR (D2O) δ 2.52 (t, 2H, J=7.2 Hz), 2.92 (t, 2H, J=7.2 Hz), 7.16 (s, 1H), 8.49 (s, 1H). [0381] Preparation of 3-(1H-Imidazol-4-yl)-propionic Acid Methyl Ester: [CHEMMOL-00065] [0382] A solution of 3-(1H-imidazol-4-yl)-propionic acid (1.95 g, 13.9 mmol) and H2SO4 (catalytic) in MeOH (30 mL) was heated at reflux for 15 h, then concentrated in vacuo. The residue was dissolved in CH2Cl2 (40 mL) and washed with saturated NaHCO3 (aq) (30 mL). The aqueous phase was saturated with sodium chloride and extracted with EtOAc (4×25 mL). The combined organic phases were dried (MgSO4) and concentrated in vacuo to give a yellow oil (1.93 g, 90%). 1H NMR (CDCl3) δ 2.68 (t, 2H, J=7.2 Hz), 2.93 (t, 2H, J=7.2 Hz), 3.69 (s, 3H), 6.81 (s, 1H), 7.55 (s, 1H). [0383] Preparation of 4-(3-Hydroxy-propyl)-imidazole-1-carboxylic Acid Tert-Butyl Ester: [CHEMMOL-00066] [0384] To a solution of 3-(1H-imidazol-4-yl)-propionic acid methyl ester (1.92 g, 12.5 mmol) in THF (25 mL) was added LiAlH4 (1.0 M/THF, 12.5 mL, 12.5 mmol) at 0° C., and the mixture was stirred at 0° C. for 15 minutes. To the mixture was added H2O (0.50 mL) followed by 15% NaOH(aq) (0.50 mL) and H2O (1.5 mL). The mixture was allowed to warm to room temperature then filtered and concentrated in vacuo to give a colourless oil (930 mg). [0385] To a solution of the crude alcohol from above (930 mg) in THF (25 mL) was added di-t-butyl dicarbonate (2.40 g, 11.0 mmol), and the solution was stirred at room temperature for 3 days. The solution was concentrated in vacuo, and the crude material was purified by column chromatography on silica gel (200:5:1 CH2Cl2/MeOH/NH4OH) to give colourless crystals (1.04 g, 37%). 1H NMR (CDCl3) δ 1.61 (s, 9H), 1.89 (m, 2H), 2.69 (t, 2H, J=6.9 Hz), 2.98 (t, 1H, J=5.7 Hz), 3.73 (dd, 2H, J=12, 5.7 Hz), 7.10 (s, 1H), 7.99 (s, 1H). [0386] Preparation of 4-(3-Oxo-propyl)-imidazole-1-carboxylic Acid Tert-Butyl Ester: [CHEMMOL-00067] [0387] To a solution of 4-(3-hydroxy-propyl)-imidazole-1-carboxylic acid tert-butyl ester (95 mg, 0.42 mmol) in CH2Cl2 (4 mL) was added Dess-Martin periodinane (214 mg, 0.505 mmol) at room temperature. After stirring at room temperature for 1 h, the mixture was diluted with EtOAc (20 mL), washed with 1 N NaOH(aq) (2×10 mL) and brine (10 mL), then dried (MgSO4) and concentrated in vacuo to give a colourless oil (86 mg, 91%). 1H NMR (CDCl3) δ 1.61 (s, 9H), 2.86 (m, 4H), 7.11 (s, 1H), 7.99 (s, 1H), 9.84 (s, 1H). [0388] Using General Procedure B: To a stirred solution of 2-[(5,6,7,8-tetrahydro-quinolin-8-ylamino)-methyl]-benzoimidazole-1-carboxylic acid tert-butyl ester (145 mg, 0.383 mmol) and 4-(3-oxo-propyl)-imidazole-1-carboxylic acid tert-butyl ester (86 mg, 0.38 mmol) in THF (4 mL) was added NaBH(OAc)3 (244 mg, 1.15 mmol) and the mixture was stirred at room temperature for 16 h. Purification of the crude material by column chromatography on silica gel (200:5:1 CH2Cl2/MeOH/NH4OH) afforded a colourless oil (39 mg, 17%). 1H NMR (CDCl3) δ 1.64 (m, 20H), 1.92 (m, 2H), 2.14 (m, 1H), 2.44 (m, 2H), 2.60-2.92 (m, 5H), 4.26 (dd, 1H, J=9.5, 5.9 Hz), 4.52 (d, 1H, J=16 Hz), 4.66 (d, 1H, J=16 Hz), 6.85 (d, 1H, J=0.9 Hz), 6.95 (dd, 1H, J=7.5, 4.8 Hz), 7.27 (m, 3H), 7.69 (m, 1H), 7.80 (m, 1H), 7.88 (d, 1H, J=1.2 Hz), 8.37 (dd, 1H, J=4.5, 1.2 Hz). [0389] A solution of 2-{[[3-(1-tert-butoxycarbonyl-1H-imidazol-4-yl)-propyl]-(5,6,7,8-tetrahydro-quinolin-8-yl)-amino]-methyl}-benzoimidazole-1-carboxylic acid tert-butyl ester (39 mg, 0.066 mmol) in 3:1 TFA/CH2Cl2 (4 mL) was stirred at room temperature for 30 minutes then concentrated in vacuo. The residue was partitioned between CH2Cl2 (15 mL) and 1 N NaOH(aq) (10 mL), and the aqueous phase was extracted with CH2Cl2 (15 mL). The combined organic extracts were dried (MgSO4) and concentrated in vacuo to afford COMPOUND 38 as a yellow foam (24 mg, 80%). 1H NMR (CDCl3) δ 1.67 (m, 3H), 1.86 (m, 1H), 2.00 (m, 1H), 2.16 (m, 1H), 2.42-2.87 (m, 6H), 4.01 (m, 3H), 6.51 (s, 1H), 7.15 (m, 3H), 7.42 (m, 2H), 7.53 (m, 2H), 8.54 (d, 11H, J=3.6 Hz); 13C NMR (CDCl3) δ 21.66, 23.85, 24.01, 28.39, 29.50, 49.64, 50.78, 62.36, 115.22, 118.74, 122.23, 122.74, 134.47, 135.25, 135.58, 138.03, 139.02, 146.88, 156.37, 157.69. ES-MS m/z 387 (M+H). Anal. Calcd. for C23H26N6.0.4CH2Cl2.0.9CH4O: C, 64.96; H, 6.82; N, 18.70. Found: C, 65.13; H, 6.93; N, 18.91.
References:
Bridger, Gary;Skerlj, Renato;Kaller, Ai;Harwig, Curtis;Bogucki, David;Wilson, Trevor R.;Crawford, Jason;McEachern, Ernest J.;Atsma, Bem;Nan, Siqiao;Zhou, Yuanxi;Schols, Dominique;Smith, Christopher Dennis;Di Fluri, Maria Rosaria US2003/220341, 2003, A1 Location in patent:Page/Page column 36-37
70346-51-9
8 suppliers
inquiry

31434-93-2
62 suppliers
inquiry

54260-89-8
5 suppliers
inquiry

31434-93-2
62 suppliers
inquiry
3465-72-3
56 suppliers
$29.00/1g

31434-93-2
62 suppliers
inquiry
![methyl 3-[1H-imidazol-4-yl]propenoate hydrochloride salt](/CAS/20180808/GIF/52838-22-9.gif)
52838-22-9
0 suppliers
inquiry

31434-93-2
62 suppliers
inquiry