
4-CHLORO-8-(TRIFLUOROMETHYL)QUINOLINE-3-CARBOXYLIC ETHYL ESTER synthesis
- Product Name:4-CHLORO-8-(TRIFLUOROMETHYL)QUINOLINE-3-CARBOXYLIC ETHYL ESTER
- CAS Number:31602-11-6
- Molecular formula:C13H9ClF3NO2
- Molecular Weight:303.66

31601-87-3
5 suppliers
inquiry

31602-11-6
33 suppliers
inquiry
Yield:31602-11-6 76%
Reaction Conditions:
Stage #1: 3-carbethoxy-8-trifluoromethyl-4-quinolonewith trichlorophosphate for 12 h;Heating / reflux;
Stage #2: with sodium hydroxide in water;
Steps:
3.3; 3
Phosphorus oxychloride (26.8 g, 175 mmol, 10 eq) was added to the compound 18 (5 g, 17.5 mmol, 1 eq) and the mixture was stirred under reflux for 12 hours . The completion of the reaction was confirmed by thin-layer chromatography. The reaction mixture was cooled, neutralized with 20% NaOH and extracted with chloroform. The extract was evaporated under reduced pressure and purified by column chromatography (ethyl acetate : hexane = 1 : 4) to obtain the target compound 19 (4 g, yield = 76 %) .1H NMR (CDCl3, 500 MHz) δ: 9.36 (s, IH), 8.65 (d, J = 8.5 Hz, IH), 8.28 (d, J = 7.3 Hz, IH), 7.77 (t, J = 7.9 Hz, IH), 4.54 (q, J = 7.1 Hz, IH), 1.47 (d, J = 7.1 Hz, 3H) .
References:
WO2008/93940,2008,A1 Location in patent:Page/Page column 67-69
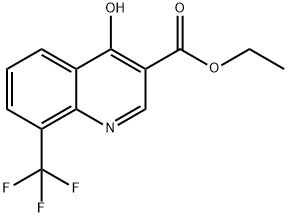
23851-84-5
75 suppliers
$10.00/1g

31602-11-6
33 suppliers
inquiry
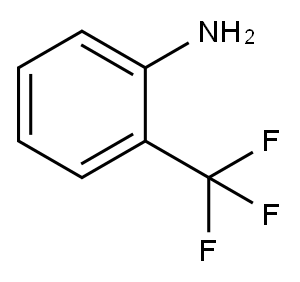
88-17-5
363 suppliers
$14.00/25g

31602-11-6
33 suppliers
inquiry
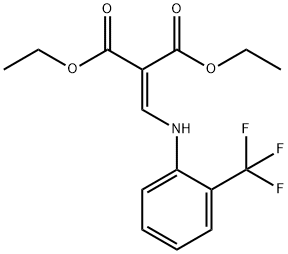
23779-94-4
9 suppliers
inquiry

31602-11-6
33 suppliers
inquiry
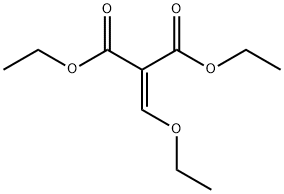
87-13-8
426 suppliers
$5.00/5g

31602-11-6
33 suppliers
inquiry