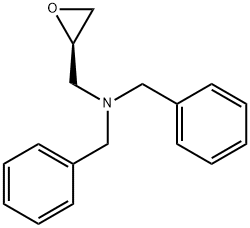
2-Oxiranemethanamine, N,N-bis(phenylmethyl)-, (2R)- synthesis
- Product Name:2-Oxiranemethanamine, N,N-bis(phenylmethyl)-, (2R)-
- CAS Number:316157-42-3
- Molecular formula:C17H19NO
- Molecular Weight:253.34
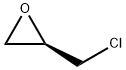
51594-55-9
364 suppliers
$10.00/1g
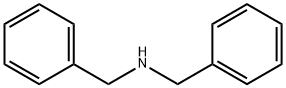
103-49-1
442 suppliers
$5.00/25g

316157-42-3
14 suppliers
inquiry
Yield:316157-42-3 97%
Reaction Conditions:
with sodium hydroxide in water;isopropyl alcohol at 10 - 25;Large scale;
Steps:
2 Method B (one pot procedure from (7/ )-(- )-epi chi oroh vdri n ) :
To a vessel, add dibenzylamine (152 kg, 770 mol) and 2-propanol (121 kg) and stir the mixture at 10-20 °C. Slowly add -epichlorohydrin (105 kg, 1135 mol) and stir the mixture at 10-20 °C until the reaction reaches <1% epichlorohydrin remaining. Slowly add 20% w/w sodium hydroxide in water (183 kg, 915 mol NaOH) and stir the mixture at 20-25 °C. Add another portion of 20% w/w sodium hydroxide in water (20 kg, 100 mol NaOH) and stir the mixture at 20-25 °C until there is <0.5% intermediate remaining. Separate the layers and concentrate the organic layer under reduced pressure to approximately 200 L remaining volume. Add EtOAc (688 kg), concentrate the mixture again under reduced pressure to approximately 200 L remaining volume, then add EtOAc (683 kg) and water (460 kg). Stir the mixture, then settle, and separate the aqueous layer. Add another portion of water (461 kg) to the organic layer. Stir the mixture, then settle, and separate the aqueous layer. Add another portion of water (451 kg) to the organic layer. Stir the mixture, then settle, and separate the aqueous layer. Concentrate the organic layer under reduced pressure to approximately 130 L remaining, then add /cvV-butanol (595 kg). Concentrate the mixture under reduced pressure to approximately 130 L remaining. Dilute the solution with te/7-butanol (167 kg) to give a solution of the title compound (373.4 kg solution, 50.8 wt%, 189.7 kg of title compound, 749 mol, 97% yield). HPLC retention time for the title compound is 14.0 min [HPLC: Waters XBridge C18 (4.6 x 150 mm, 3.5 pm), 35 °C, 1.2 mL/min, 215 nm detection, gradient: 5-95% B in 16 min, hold to 18 min, 5% B at 18.1 min; Solvent A = 10 mM ammonium formate, pH 9.0, in purified water, Solvent B = ACN]
References:
WO2021/118906,2021,A1 Location in patent:Page/Page column 14-15

103-49-1
442 suppliers
$5.00/25g

316157-42-3
14 suppliers
inquiry