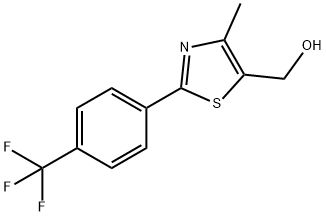
(4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOL-5-YL)METHANOL synthesis
- Product Name:(4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOL-5-YL)METHANOL
- CAS Number:317318-96-0
- Molecular formula:C12H10F3NOS
- Molecular Weight:273.27
![Ethyl 4-methyl-2-[4-(trifluoromethyl)phenyl]-1,3-thiazole-5-carboxylate](/CAS/GIF/175277-03-9.gif)
175277-03-9
120 suppliers
$15.00/5g
![(4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOL-5-YL)METHANOL](/CAS/GIF/317318-96-0.gif)
317318-96-0
67 suppliers
$65.00/50mg
Yield:317318-96-0 97%
Reaction Conditions:
Stage #1: 4-methyl-2-[4-(trifluoromethyl)phenyl]thiazole-5-carboxylic acid ethyl esterwith lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 1.66667 h;
Stage #2: with water in tetrahydrofuran;
Steps:
1.1
To a solution of lithium aluminum hydride (10 mL of 1.0 M solution in5 THF, 10.0 mmol) in THF (20 mL) at 0°C, was added a solution of 4-methyl-2-(4-trifluoromethyl-phenyl)-thiazole-5-carboxylic acid ethyl ester (3.0 g, 9.5 mmol) in dry THF (30 mL). After stirring at 0°C for 10 min, the reaction mixture was warmed up to room temperature and continued to stir for 1.5 h. The reaction was quenched by slow addition of water (3 mL), IN NaOH (40 mL). The resulting mixture was filtered10 through Celite and the filtrate was extracted with ethyl acetate (50 mL X 2). The combined organic solution was washed with brine and dried over Na2SC>4. After removal of solvent, 2.51 g (97% yield) of the desired product was obtained as a bright yellow solid. .H NMR (400 MHz, CDC13), 5 (ppm): 8.01 (d, 2H), 7.67 (d, 2H), 4.85 (s, 2H), 2.47 (s, 3H).
References:
WO2005/60958,2005,A1 Location in patent:Page/Page column 115-116
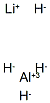
16853-85-3
405 suppliers
$14.00/1g
![(4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOL-5-YL)METHANOL](/CAS/GIF/317318-96-0.gif)
317318-96-0
67 suppliers
$65.00/50mg
![4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]THIAZOLE-5-CARBOXYLIC ACID](/CAS/GIF/144059-86-9.gif)
144059-86-9
85 suppliers
$15.00/1g
![(4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOL-5-YL)METHANOL](/CAS/GIF/317318-96-0.gif)
317318-96-0
67 suppliers
$65.00/50mg
![5-Thiazolecarboxylic acid, 4-ethyl-2-[4-(trifluoromethyl)phenyl]-, ethyl ester](/CAS/20210305/GIF/853076-90-1.gif)
853076-90-1
0 suppliers
inquiry
![(4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOL-5-YL)METHANOL](/CAS/GIF/317318-96-0.gif)
317318-96-0
67 suppliers
$65.00/50mg
![5-Thiazolemethanol, 4-bromo-2-[4-(trifluoromethyl)phenyl]-](/CAS/20210305/GIF/873066-26-3.gif)
873066-26-3
0 suppliers
inquiry
594-27-4
112 suppliers
$31.00/5g
![(4-METHYL-2-[4-(TRIFLUOROMETHYL)PHENYL]-1,3-THIAZOL-5-YL)METHANOL](/CAS/GIF/317318-96-0.gif)
317318-96-0
67 suppliers
$65.00/50mg