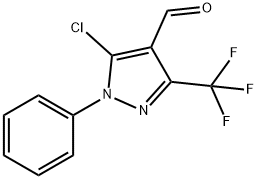
3-trifluoromethyl-1-phenyl-1H-5-chloropyrazole-4-carbaldehyde synthesis
- Product Name:3-trifluoromethyl-1-phenyl-1H-5-chloropyrazole-4-carbaldehyde
- CAS Number:318288-78-7
- Molecular formula:C11H6ClF3N2O
- Molecular Weight:274.63
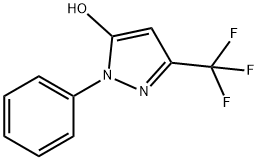
96145-98-1
77 suppliers
$16.00/250mg

318288-78-7
17 suppliers
$173.19/1gm:
Yield:318288-78-7 79.1%
Reaction Conditions:
with trichlorophosphate in DMF (N,N-dimethyl-formamide) at 0 - 20; for 1 h;Heating / reflux;
Steps:
10 Production of 5-chloro-1-phenyl-3-trifluoromethyl-1H-pyrazole-4-carboaldehyde
33.6 g (219.1 mmoles) of phosphorus oxychloride was added to 7.7 g (105.2 mmoles) of N,N-dimethylformamide with ice-cooling. Thereto was added, at room temperature, 20 g (87.7 mmoles) of 1-phenyl-3-trifluoromethyl-1H-pyrazol-5-ol. The resulting mixture was refluxed for 1 hour with heating, to give rise to a reaction. After the completion of the reaction, the reaction mixture was poured into water with ice-cooling, followed by extraction with chloroform. The resulting organic layer was washed with an aqueous sodium hydrogencarbonate solution and an aqueous sodium chloride solution in this order and then dried over anhydrous magnesium sulfate. The resulting solution was subjected to vacuum distillation to remove the solvent contained therein. The residue was purified by silica gel column chromatography (developing solvent: hexane-ethyl acetate mixed solvent) to obtain 19.1 g (yield: 79.1%) of 5-chloro-1-phenyl-3-trifluoromethyl-1H-pyrazole-4-carboaldehyde as white crystals. 1H-NMR [CDCl3/TMS, δ (ppm)]: 10.06 (1H,s), 7.57 (5H,s)
References:
EP1364946,2003,A1 Location in patent:Page/Page column 192
100-63-0
357 suppliers
$10.00/1g

318288-78-7
17 suppliers
$173.19/1gm:
59-88-1
355 suppliers
$15.00/10g

318288-78-7
17 suppliers
$173.19/1gm: