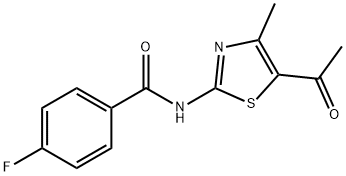
N-(5-acetyl-4-methylthiazol-2-yl)-4-fluorobenzamide synthesis
- Product Name:N-(5-acetyl-4-methylthiazol-2-yl)-4-fluorobenzamide
- CAS Number:321532-91-6
- Molecular formula:C13H11FN2O2S
- Molecular Weight:278.3
Yield:321532-91-6 62%
Reaction Conditions:
with triethylamine in dichloromethane;Reflux;
Steps:
2.1a General procedure for the synthesis of N-(5-acetyl-4-methylthiazol-2-yl) Arylamide derivatives:
General procedure: 3-chloropentane-2,4-dione (1.0 equiv., 0.013 mmol)was refluxed with thiourea for 6-7 h (MeOH/H) whichyielded 1-(2-amino-4-methylthiazol-5-yl)ethenone andthen the yielded product was further refluxed with(Benzoyl chloride(R)) for 23 h (Et 3N CH2Cl2) whichlead to the formation of a parent compound that was(N-(5-acetyl-4-methylthiazol-2-yl)-4-(substituted)benzamide)(Table 1).
References:
Channar, Kashif Ali;Channar, Pervaiz Ali;Ejaz, Syeda Abida;Indher, Hafiz Abdul Bari;Ismail, Hammad;Mahmood, Hafiz Mohammad Kashif;Rafiq, Mamoona;Saeed, Aamer;Saeed, Amna;Saeed, Shomaila;Ujan, Rabail [Journal of Chemical Sciences,2022,vol. 134,# 1,art. no. 7]
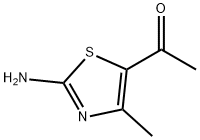
30748-47-1
187 suppliers
$18.00/1g
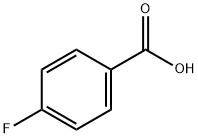
456-22-4
468 suppliers
$10.00/100g

321532-91-6
6 suppliers
inquiry
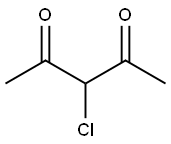
1694-29-7
99 suppliers
$40.00/10g

321532-91-6
6 suppliers
inquiry
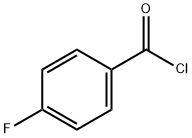
403-43-0
448 suppliers
$9.00/25g

321532-91-6
6 suppliers
inquiry
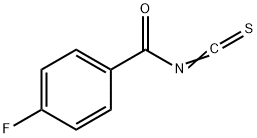
78225-74-8
7 suppliers
inquiry

321532-91-6
6 suppliers
inquiry