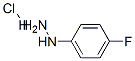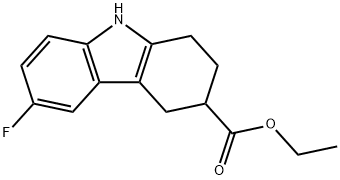
6-FLUORO-2,3,4,9-TETRAHYDRO-1H-CARBAZOLE-3-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:6-FLUORO-2,3,4,9-TETRAHYDRO-1H-CARBAZOLE-3-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:322725-63-3
- Molecular formula:C15H16FNO2
- Molecular Weight:261.29
Yield:322725-63-3 98%
Reaction Conditions:
in ethanol; for 16 h;Product distribution / selectivity;Heating / reflux;Fischer Indole Synthesis;
Steps:
2; 4.1; 11
[0097] Example 2: N-[(6-fluoro-2,3,4,9-tetrahydro-lH-carbazol-3-yl)methyl]-N-{[(2S)-8- methyl-2,3-dihydro[l,4]dioxino[2,3-f]quinolin-2-yl]methyl}amine6-Fluoro-23 A9-tetrahydro-lH-carbazole-3-carboxylic acid ethyl ester (III) [0098] 4-Cyclohexanonecarboxylic acid ethyl ester (25 g, 0.14 m) and 4- fluorophenyhydrazine hydrochloride (22.5 g, 0.13 m) were dissolved in ethanol (450 mL) and heated under reflux for 16 hrs. After cooling, the white solid was filtered off and the solvent removed under reduced pressure. After partitioning the residue between water and ethyl acetate, the organic portion was separated, dried (MgSO4), and evaporated under reduced pressure to give compound III ( 35.5 g, 0.13 m). The crude product was recrystallized from heptane, mp: 115-117βC. MS: [M+H]+(at)m/e=262. [Lit. ref.: Block, M. H., et al. J Med. Chem. 2002, 45, 3509].; [0104] Example 4: S,-S-(6-Fluoro-2,3,4,9-tetrahydro-lH-carbazol-3-ylmethyl)-(8-methyl- 2,3-dihydro-[l,4]dioxino[2,3-flquinolin-2ylmethyl)-amine[0105] The title compound can be made by the following procedure. Step 1 : 6-Fluoro-2,3,4,9-tetrahydro-lH-carbazole-3-carboxylic acid ethyl ester [0106] 4-Cyclohexanonecarboxylic acid ethyl ester (77 g, 450 mmol) and 4- fluorophenylhydrazine hydrochloride (72 g, 443 mmol) were refluxed in 1.25 L of anhydrous ethanol overnight. The resulting yellow solution was cooled, crystals filtered, the filtrate evaporated, the residue partitioned between ethyl acetate and water, organic layer dried over sodium sulfate, evaporated, crystallized from ethyl acetate/heptane to give 113 g (98%) of the desired product.
References:
WO2006/89053,2006,A2 Location in patent:Page/Page column 34-35; 28