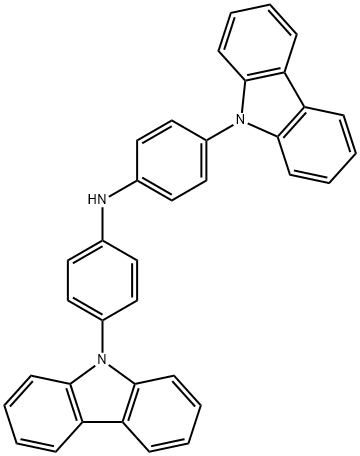
Benzenamine, 4-(9H-carbazol-9-yl)-N-[4-(9H-carbazol-9-yl)phenyl]- synthesis
- Product Name:Benzenamine, 4-(9H-carbazol-9-yl)-N-[4-(9H-carbazol-9-yl)phenyl]-
- CAS Number:325492-24-8
- Molecular formula:C36H25N3
- Molecular Weight:499.6
Yield:325492-24-8 96%
Reaction Conditions:
with tris-(dibenzylideneacetone)dipalladium(0);sodium t-butanolate;tert-butyl XPhos in o-xylene at 140; for 15 h;Inert atmosphere;
Steps:
36 Example-36
0.327 g (1.00 mmol) of di (4-bromophenyl) amine, 0.352 g (2.10 mmol) of carbazole, 9.16 mg (10.0 μmol) of tris (dibenzylideneacetone) dipalladium, 29.8 mg (70.0 μmol) of 2- (di-tert-butylphosphino) -2 ', 4', 6'-triisopropyl-1,1'-biphenyl, 0.288 g (3.00 mmol) of sodium tert-butoxide and 6 mL of o-xylene were added, and the inside of the reaction system was purged with argon gas.Thereafter, the mixture was heated to 140 ° C. and stirred for 15 hours. After cooling to room temperature, 25 mL of water was added to quench. Further 20 mL of ethyl acetate was added and the mixture was stirred until homogeneous, then 0.2 mL of ethyl acetate layer was collected, diluted with 4 mL of tetrahydrofuran, And analyzed by HPLC. As a result, the conversion of di (4-bromophenyl) amine was 99% or more, It was confirmed that bis [4- (9H-carbazol-9-yl) phenyl] amine was formed in 96% relative to di (4-bromophenyl) amine.
References:
JP2018/90563,2018,A Location in patent:Paragraph 0051; 0053; 0115-0116

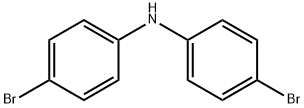
52708-37-9
63 suppliers
$394.00/1G
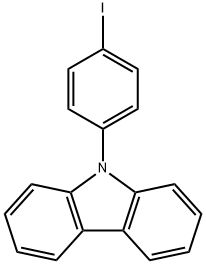
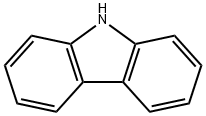
57103-15-8
85 suppliers
$20.00/100mg
![Benzenamine, 4-(9H-carbazol-9-yl)-N-[4-(9H-carbazol-9-yl)phenyl]-](/CAS/20210305/GIF/325492-24-8.gif)
325492-24-8
0 suppliers
inquiry