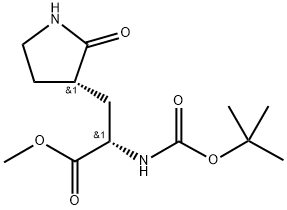
Methyl (S)-2-(Boc-amino)-3-[(S)-2-oxo-3-pyrrolidinyl]propanoate synthesis
- Product Name:Methyl (S)-2-(Boc-amino)-3-[(S)-2-oxo-3-pyrrolidinyl]propanoate
- CAS Number:328086-60-8
- Molecular formula:C13H22N2O5
- Molecular Weight:286.33
![L-Glutamic acid, 4-(2-aminoethyl)-N-[(1,1-dimethylethoxy)carbonyl]-, 1,5-dimethyl ester, (4S)-](/CAS/20210305/GIF/328086-58-4.gif)
328086-58-4
2 suppliers
inquiry
![Methyl (S)-2-(Boc-amino)-3-[(S)-2-oxo-3-pyrrolidinyl]propanoate](/CAS/20210111/GIF/328086-60-8.gif)
328086-60-8
216 suppliers
inquiry
Yield:328086-60-8 90%
Reaction Conditions:
with triethylamine in tetrahydrofuran; for 8 h;Reflux;
Steps:
1-2
The intermediate 2 prepared above was dissolved in 40.0 ml of tetrahydrofuran, added with 10.0 ml of triethylamine, heated under reflux for 8 hours, and then cooled to room temperature. 30.0 ml of saturated saline was added for quenching. The layers were left to stand, and the organic layer was dried and concentrated. The crude product was purified by column chromatography to obtain 4.68 g of the product with a yield of 90.0%. The product is: (S)-2-(Boc-amino)-3-[(S)-2-oxo-3-pyrrolidinyl]propionic acid methyl ester.
References:
CN114605301,2022,A Location in patent:Paragraph 0032; 0036; 0037; 0038; 0040-0042
328086-57-3
46 suppliers
inquiry
![Methyl (S)-2-(Boc-amino)-3-[(S)-2-oxo-3-pyrrolidinyl]propanoate](/CAS/20210111/GIF/328086-60-8.gif)
328086-60-8
216 suppliers
inquiry

56-86-0
790 suppliers
$5.00/25g
![Methyl (S)-2-(Boc-amino)-3-[(S)-2-oxo-3-pyrrolidinyl]propanoate](/CAS/20210111/GIF/328086-60-8.gif)
328086-60-8
216 suppliers
inquiry

24424-99-5
824 suppliers
$13.50/25G
![Methyl (S)-2-(Boc-amino)-3-[(S)-2-oxo-3-pyrrolidinyl]propanoate](/CAS/20210111/GIF/328086-60-8.gif)
328086-60-8
216 suppliers
inquiry
![L-Glutamic acid, N-[(1,1-dimethylethoxy)carbonyl]-4-(2-propen-1-yl)-, 1,5-dimethyl ester, (4S)-](/CAS/20210305/GIF/213475-89-9.gif)
213475-89-9
1 suppliers
inquiry
![Methyl (S)-2-(Boc-amino)-3-[(S)-2-oxo-3-pyrrolidinyl]propanoate](/CAS/20210111/GIF/328086-60-8.gif)
328086-60-8
216 suppliers
inquiry