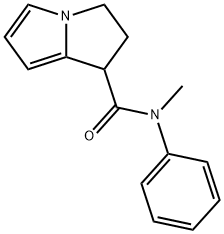
N-Methyl-N-phenyl-2,3-dihydro-1H-pyrrolizine-1-carboxaMide synthesis
- Product Name:N-Methyl-N-phenyl-2,3-dihydro-1H-pyrrolizine-1-carboxaMide
- CAS Number:328936-22-7
- Molecular formula:C15H16N2O
- Molecular Weight:240.3
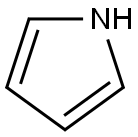
109-97-7
339 suppliers
$9.00/5G
328936-18-1
6 suppliers
inquiry
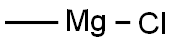
676-58-4
277 suppliers
$15.00/25ml

328936-22-7
7 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride;sodium carbonate in tetrahydrofuran;diethylene glycol dimethyl ether;toluene;
Steps:
Preparation of 4-Chloro-N-methyl-N-phenyl-2-(2-pyrrolyl)butanamide (a compound of formula 3) in Tetrahydrofuran/Toluene/Diglyme
Pyrrole (40 mL, 36.7 g, 557 mmol, 2.3 equivalents with respect to 2-bromo-4-chloro-N-methyl-N-phenylbutanamide) was added dropwise to a solution of methylmagnesium chloride in tetrahydrofuran (180 mL*2.9 M, 522 mmol, 2.1 equivalents) under a nitrogen atmosphere at a temperature of 35° C. over a period of 30 minutes. Additional tetrahydrofuran (115 mL) was added to the mixture. After the addition was complete, the suspension was stirred at 25° C. for 30 minutes and then cooled to 15° C. to 20° C. A mixture of 2-bromo-4-chloro-N-methyl-N-phenylbutanamide (76.57 g, 249 mmol), toluene (90 mL), and diglyme (50 mL) was added to the suspension in about 1 minute. The temperature increased from 30° C. to 35° C. in the first 30 minutes after addition. The resulting clear solution was stirred for 3 hours to 5 hours at room temperature and then cooled to about 5° C. Cooled hydrochloric acid (150 mL*2 N, 300 mmol), was added over 1 minute to the reaction solution. The temperature of the suspension quickly increased to 30° C. to 35° C., and it was stirred for about 5 minutes. The resultant layers were separated and the organic layer was passed through a column of sodium hydroxide pellets. The eluent layer was again separated. The combined organic layer was stirred overnight with sodium carbonate (2.64 g) at a temperature of -5° C., then distilled to recover the cosolvent and solvents. The residual dark suspension containing the 4-chloro-N-methyl-N-phenyl-2-(2-pyrrolyl)-butanarnide product was cooled to 25° C. and toluene (150 mL) was added. The resulting solution was filtered through an alumina column for decolonization and was used directly in the next step for the preparation of N-methyl-N-phenyl-2,3-dihydro-1H-pyrrolizine-1-carboxamide.
References:
US6197976,2001,B1