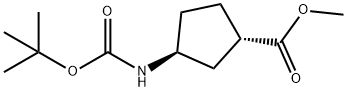
(1S,3S)-N-BOC-1-AMINOCYCLOPENTANE-3-CARBOXYLIC ACID METHYL ESTER synthesis
- Product Name:(1S,3S)-N-BOC-1-AMINOCYCLOPENTANE-3-CARBOXYLIC ACID METHYL ESTER
- CAS Number:329910-39-6
- Molecular formula:C12H21NO4
- Molecular Weight:243.3

1085842-51-8
38 suppliers
$100.00/50mg

24424-99-5
825 suppliers
$13.50/25G

329910-39-6
39 suppliers
$514.00/100mg
Yield:329910-39-6 84%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20; for 1.5 h;
Steps:
1 Step 1-methyl (1S,3S)-3-((tert-butoxycarbonyl)amino)cyclopentane-1-carboxylate
To a stirred solution of methyl (1S,3S)-3-aminocyclopentane-1-carboxylate hydrochloride (4.5 g, 25 mmol) and TEA (10.5 mL, 75 mmol) in DCM (100 mL) was dropwise added Boc-anhydride (6.6 g, 30 mmol) at 0° C. The resulting reaction mixture was then allowed to warm to rt and then stirred for 1.5 h. The reaction mixture was then transferred into ice water and the resulting mixture was extracted using DCM (3×100 mL). The combined organic layer was dried over anhydrous sodium sulfate and filtered. The filtrate was evaporated under reduced pressure and the crude product was purified using silica gel column chromatography (40% EAc-Hexanes) to give methyl (1S,3S)-3-((tert-butoxycarbonyl)amino)cyclopentane-1-carboxylate as a white solid (5.1 g, 84%). LC-MS (ESI+) m/z 261.3 (M+18)+.
References:
US2019/192668,2019,A1 Location in patent:Paragraph 2028; 2029