
METHYL 2-METHYL-6-OXO-4-[4-(TRIFLUOROMETHYL)PHENYL]-1,4,5,6-TETRAHYDRO-3-PYRIDINECARBOXYLATE synthesis
- Product Name:METHYL 2-METHYL-6-OXO-4-[4-(TRIFLUOROMETHYL)PHENYL]-1,4,5,6-TETRAHYDRO-3-PYRIDINECARBOXYLATE
- CAS Number:330216-62-1
- Molecular formula:C15H14F3NO3
- Molecular Weight:313.27
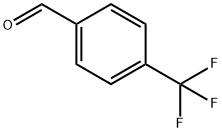
455-19-6
409 suppliers
$6.00/5g
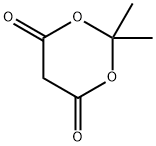
2033-24-1
628 suppliers
$6.00/25g
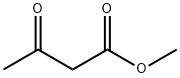
105-45-3
651 suppliers
$5.00/5g
![METHYL 2-METHYL-6-OXO-4-[4-(TRIFLUOROMETHYL)PHENYL]-1,4,5,6-TETRAHYDRO-3-PYRIDINECARBOXYLATE](/StructureFile/ChemBookStructure3/GIF/CB0766265.gif)
330216-62-1
8 suppliers
inquiry
Yield:330216-62-1 19%
Reaction Conditions:
with ammonium acetate;acetic acid for 2 h;Heating / reflux;
Steps:
5.1
Example 5; N-lH-Indazol-5-yl-2-methyl-6-oxo-4- [4- (trifluoromethyl) phenyl]- 1, 4, 5, 6-tetrahydro-3-pyridinecarboxamide.; Step 1;. Methyl 4- [4- (trifluoromethyl) phenyl]-2-methyl-6-oxo-1, 4,5, 6-tetrahydro-3- pyridinecarboxylate;. 4-Trifluoromethylbenzaldehyde (25.0 mL, 147 mmol, 1.00 equiv), 2,2- dimethyl-1, 3-dioxane-4,6-dione (21.2 g, 147 mmol, 1.00 equiv), methyl acetoacetate (15. 8 mL, 147 mmol, 1. 00 equiv), and ammonium acetate (11. 8 g, 154 mmol, 1.05 equiv) were dissolved in acetic acid (150 mL) and heated to reflux for 2 hours. Addtition of water to the stirred reaction mixture induced precipitation of a solid residue. The solid was recovered by filtration and triturated with 50% CH2Ck/hexane to afford 8. 50g (19%) of the product as a white solid. MS (ES+) m/e 300 [M+H] +.
References:
WO2005/82890,2005,A1 Location in patent:Page/Page column 30